Introduction
Urinary tract infections (UTIs) are among the most common bacterial infections in human population. Escherichia coli (E. coli) is by far the leading cause of community-acquired UTIs, with a urine sample prevalence of over 80% (Priyadharshana et al. 2019). Its emerging antimicrobial resistance, along with the existence multidrug-resistant (MDR) strains is one of the most difficult global healthcare challenges (Kahlmeter et al. 2015; Zykov et al. 2020).
The most important antimicrobial resistance mechanism of uropathogenic Escherichia coli (UPEC) is the production of antibiotic-degrading enzymes. A significant proportion of E. coli strains produce different beta lactamases, including extended-spectrum β-lactamase (ESBL), with around half of isolates being ESBL-producers. Other clinically significant beta-lactamases are plasmid-mediated AmpC, as well as carbapenemases, including OXA-48-like enzymes. Carbapenemase-producing E. coli is an emerging pathogen and a significant challenge (Zykov et al. 2020).
In Europe, including Serbia, E. coli isolates obtained from women with acute uncomplicated UTIs have increasing antimicrobial resistance, particularly to ciprofloxacin and trimethoprim-sulfamethoxazole (TMP-STX) (Kahlmeter et al. 2015; Djordjević et al. 2019). The aforementioned antibacterial agents are the first-line antibiotics for acute, uncomplicated UTIs according to the „Rational use of antibiotics“guidelines issued by the Serbian Ministry of Health in 2018 (Нови Национални Водич За Рационалну Употребу Антибиотика, 2018), as well as the already two – decades old national UTI treatment strategy in Serbia (Ministarstvo Zdravlja Republike Srbije, 2004). Therefore, as aforementioned studies are reporting an increasing resistance to these agents, the normative rationale for their use as a first-line agent is being challenged (Bollestad et al. 2018).
Consequently, with the urgent need for new UTI treatment modalities, older and less frequently used antibiotics have been tested and evaluated against MDR E. coli in numerous studies. One of these antibacterials is pivmecillinam, the oral prodrug of the penicillin derivative mecillinam (also named amdinocillin). Based on recent research, mecillinam resistance in E. coli mostly remains low in Europe (Kahlmeter et al. 2015). When mecillinam was introduced to clinical practice, E. coli was found to be the most common uropathogen that was highly susceptible to this antibiotic (Tutone et al. 2022). Therefore, in several nations, it is commonly used as a first medication for the empirical management of UTI, mostly due to its relatively low resistance rates and low damaging effects on the gut microbiota as a pro-drug and good in vitro efficacy against β-lactamase-producing E. coli (Zykov et al. 2020).
Pivmecillinam is yet to be registered in Serbia, in order to be tested as a potential first-choice antibiotic for UTI caused by E. coli; thus, we review the current literature data on mecillinam’s antibacterial effect in this particular bacteria.
2. Methodology
A systematic review on the role of mecillinam in the treatment of UTI caused by E. coli was performed. A search of the Pubmed database was conducted to identify relevant, original articles related to the aforementioned topic. Terms used for the search were: mecillinam AND E. coli. In order to focus on innovative findings, only articles published in the last 10 years were included. Studies were included if they were journal articles that reported: 1) UTIs caused by E. coli treatment with mecillinam, 2) mecillinam as an antibiotic of interest in terms of mechanism of action, sensitivity, resistance and guidelines for clinical application, 3) prevalence of E. coli resistance to mecillinam and its clinically relevant mechanisms. Exclusion criteria included: 1) reviews, abstracts, books, opinion articles, letters to the editor, 2) non-English articles. The PRISMA (Preferred Reporting Items for Systematic Reviews and MetaAnalyses) protocol was adopted in this literature review to offer the replicability of the study, as shown in Figure 1 (Page et al. 2021). Additional manuscripts of interest were identified through a manual search of the reference lists of the retrieved articles. The final text represents an overview of the antimicrobial properties of mecillinam as a first-line antibiotic in the treatment of UTIs caused by E. coli.

Figure 1. The PRISMA selection process of relevant literature
2.1. Mecillinam – mechanism of antibacterial action
Mecillinam is a β-lactam antibiotic that interfere with the essential penicillin-binding protein 2 (PBP2) (Kocaoglu & Carlson, 2015). The target of β-lactam antibiotics is the transpeptidase activity of penicillin-binding proteins (PBPs) for the synthesis of cross-links in the peptidoglycan (PG) of bacterial cell walls (Thulin & Andersson, 2019). The inhibition of PBP is identified as a crucial element contributing to the lethal effects of β-lactam antibiotics. The downstream metabolic effects resulting from the administration of mecillinam to E. coli demonstrate that the inhibition of PBP2 has a distinct outcome of inducing toxic metabolic dysfunction, leading to the disruption of the usual balance between anabolic and catabolic processes. The previously mentioned process leads to an unproductive loop of cell wall synthesis and subsequent disintegration, resulting in the depletion of cellular resources and compromising the quality regulation mechanism for enzymes responsible for breaking bonds in the cell wall structure, ultimately posing an obstacle to the integrity of PG (Cho et al. 2014). The maintenance of PG homeostasis is closely associated with the activation levels of the Cpx system, which is a stress response system located in the cell envelope. The activation of the Cpx system offers cellular protection against antibiotics that block PG production. Fortunately, the usual insufficient activation of Cpx leads to aberrant cellular structures that exhibit a greater susceptibility to β-lactam antibiotics, and substantial deficiencies in cell division and growth. These observations are in line with a disruption in the maintenance of PG homeostasis (Delhaye et al. 2016).
2.2. Mecillinam for the UTI treatment
As an old penicillin derivative, mecillinam was introduced worldwide in national and international protocols for UTI treatment in ambulatory settings after showing high efficiency for uncomplicated low UTIs. In Germany, this drug was re-introduced in 2016, as a first-line treatment of acute uncomplicated cystitis (Kresken et al. 2022). In 2019, pivmecillinam was the recommended first-choice antibiotic used to treat UTIs in Denmark (Nielsen et al. 2019). Along with fosfomycin and nitrofurantoin, pivmecillinam is a first-line treatment for uncomplicated cystitis in women according to the newest European Association of Urology (EAU) guidelines published in 2023 (EAU Guidelines, 2023).
Dosing regimens of pivmecillinam that are proven to be effective in UTI treatment are 200 mg and 400 mg, three times daily. Against clinical isolates of E. coli, including multidrug-resistant (MDR) ones, these dosages showed high effectiveness in bladder localized infections (Zykov et al. 2020). An uncontrolled treatment trial documented that pivmecillinam of 400 mg, given for 1 week following 3 days of parenteral antibiotics, is a suitable treatment option in patients suffering from bacteriemic UTI due to E. coli (Hansen et al. 2022). In comparison, timocillin, also a PBP2-specific beta-lactam showed low efficiency for complications such as systemic infection (Chen et al. 2016), further highlighting the need for randomised clinical trials studying the efficacy of pivmecillinam as the standard of care for febrile UTI. It was also experimentally demonstrated that there was a highly significant antimicrobial effect of mecilinam in the bladder, as well as in the kidney, showing its high potential for upper UTI treatment (Zykov et al. 2020).
As no differences in resistance rates were observed in older patients, this patient population does not require specific treatment protocols for uncomplicated UTIs, but recommendations based on gender seem to be needed. It was registered that E. coli was significantly more common in females than in males, with other bacterial species being more common in male UTIs, with a tendency for gender-dependent resistance patterns (Fagan et al. 2015). New prescriptions of UTI antibiotics and relapse within 3 months after completion of therapy were more frequent with pivmecillinam than trimethoprim in treating lower UTIs in men. Nevertheless, it still has acceptable efficiency for empirical treatment (Montelin et al. 2019).
It is a general knowledge that the odds of resistance to any antibiotic increase significantly after recent exposure to it, more precisely within 90 days prior to the UTI episode. Mecillinam showed the lowest increase in the odds for selection of resistance overall (Jensen et al. 2022). so that leaves it as a valuable treatment option even in recurrent UTI.
2.3. Mecillinam as a treatment option for MDR, β-lactamase-producing E. coli isolates
An increasing resistance of E. coli to different antimicrobial agents has been noticed. Most widespread antibiotics against which the resistance of E. coli is prevalent are the following: ampicillin (21.0-53.7%), amoxicillin (30-43.3%), amoxicillin-clavulanic acid (16.7-20.5%), and cefuroxime (11.3-13.5%). Looking at two commonly used antibiotics for UTI treatment, E. coli also exhibits a highly prevalent resistance to TMP-STX (16.1-31.6%) and ciprofloxacin (5.2-32.3%) (Table 1). This is especially significant when compared to overall low mecillinam resistance (0-10.8%) (Sundvall et al. 2014; Bollestad et al. 2018; Ny et al. 2019; Kresken et al. 2022; Plambeck et al. 2022; Stoltidis-Claus et al. 2023).
Table 1. Prevalence of E. coli resistance (%) to most frequently prescribed antibiotics for UTIs treatment in different European countries compared to Mecillinam.
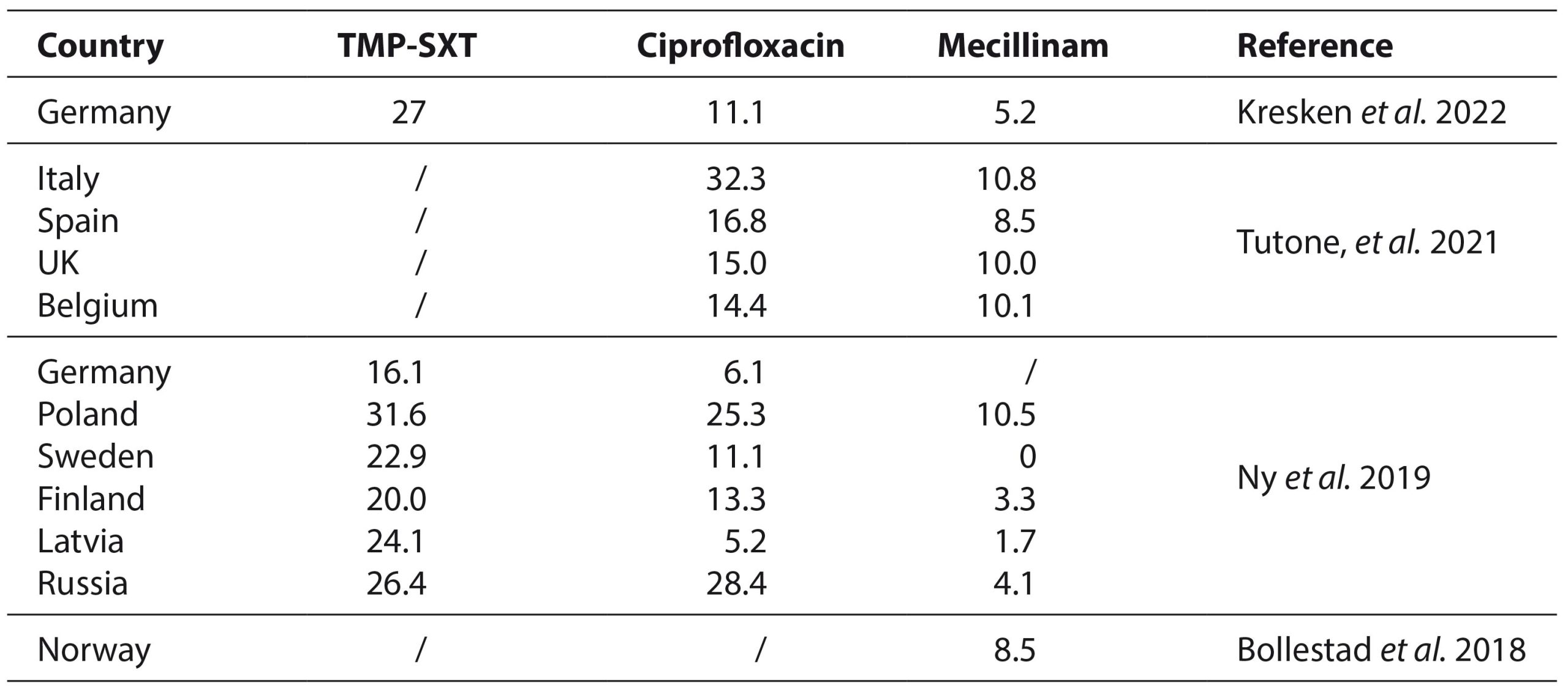
TMP-SXT, trimethoprim-sulfamethoxazole; UK, United Kingdom
In Serbia, during the pre-COVID era, E. coli isolated from community-acquired UTI cases exhibited higher prevalence of resistance to TMP-STX (37.46%) and ciprofloxacin (23.80%) to those shown in Table 1, respectively (Djordjević et al. 2019). Also, MDR is noted to be most prevalent in isolates showing high-level ciprofloxacin resistance (Strand et al. 2014). This is of high importance, as both ciprofloxacin and especially trimethoprim-sulfamethoxazole are key antibiotics in Serbian UTI treatment guidelines (Нови Национални Водич За Рационалну Употребу Антибиотика, 2018). During the COVID-19 pandemic in Serbia, the overall antimicrobial resistance, has escalated, especially in Gram-negative bacteria, including uropathogenic isolates (Gajic et al. 2023). This could potentially indicate that E. coli resistance prevalence is a lot higher in 2023, a post-COVID period. This could provide a further support for introduction of mecillinam in Serbia, as well as testing it for UTI treatment afterwards.
Mecillinam demonstrates potent antimicrobial activity against strains that produce plasmid-mediated enzymes (AmpCs) (Zykov et al. 2020), and ESBLs of the CTX-M family (Plambeck et al. 2022; Zykov et al. 2020). This is significant because the CTX-M mutation is the most prevalent mutation in ESBL E. coli (Priyadharshana et al. 2019). Despite this, mecillinam resistance resulting from the CTX-M mutation remains uncommon (Nielsen et al. 2019). The quantities of mecillinam required to inhibit 50% and 90% of the ESBL-producing isolates, in comparison to other isolates, were nearly equivalent, hence emphasizing its anti-ESBL activity (Kresken et al. 2022). Thus, mecillinam maintains its status being a carbapenem-sparing drug (O’Kelly et al. 2016; Fuchs & Hamprecht, 2019; Farfour et al. 2022). This fact supports the recommendation to regard pivmecillinam as a first-line option for the treatment of uncomplicated lower UTIs in adults (Kresken et al. 2022), as well as in pediatric population (Thaulow et al. 2021).
Further evidence show that mecillinam exhibits high antimicrobial activity against MDR E. coli strains, including β-lactamase producing ones. (Fuchs & Hamprecht, 2019) Such is the case in metallo-β-lactamase (MBL)-producing isolates (both NDM-1 and IMP types) and OXA-48-like-positive strains (Samuelsen et al. 2017; Fuchs et al. 2020; Zykov et al. 2020; Emeraud et al. 2022; Plambeck et al. 2022), even in those with high carbapenem MICs (minimal inhibitory concentrations) (Fuchs et al. 2020).
2.4. Mecillinam and other antibiotics as a treatment choice for highly resistant E. coli isolates
In European countries, 80-97.4% of clinical E. coli isolates tend to show high sensitivity to mecillinam, based on the recent studies (Samuelsen et al. 2017; Fuchs & Hamprecht, 2019; Priyadharshana et al. 2019; Emeraud et al. 2022; Tutone et al. 2022). However, certain authors reported MDR E. coli associated with ESBL-producing plasmids that have become a major problem, especially if they are also mecillinam-resistant with a CTX-M-15 mutation. Further investigation might show if a drug combination could be a potential solution for limiting resistance mutations. (Rosenkilde et al. 2019).
Mecillinam combined with ceftazidime/avibactam or avibactam only substantially reduced MICs for almost all evaluated strains, making them susceptible to this combination. Mecillinam in combination with avibactam or ceftazidime/avibactam has a notable effect on most types of MDR Gram-negative bacteria both in vitro and in vivo (List et al. 2022). This combination could be a new efficient antibiotic treatment against multi-drug-resistant carbapenemase-producing E coli, resistant to individual treatment regimes. The combination of trimethoprim and mecillinam shown restricted synergy in specific instances, whereas mecillinam and nitrofurantoin combinations showed antagonistic interactions against all tested strains (Fatsis-Kavalopoulos et al. 2020), hence application of both combinations should be reconsidered in clinical practice. Mecillinam-resistant isolates exhibited a cross-resistance to temocillin (same antibiotic group), and were more likely to have cross-sensitivity towards drugs from other groups, such as azithromycin and chloramphenicol (Podnecky et al. 2018). A comprehensive examination was carried out by researchers on antibiotic combinations involving mecillinam. The study specifically highlighted the remarkable effectiveness of a triple combination comprising of fosfomycin, a highly potent treatment for multi-drug resistant uropathogens (Chen et al. 2016), along with aztreonam and mecillinam in significantly reducing bacterial populations (Hickman et al. 2014).
2.5. Treatment options for Mecillinam-resistant E. coli isolates
In laboratory settings, the prevalence of mutations leading to E. coli mecillinam resistance is reported to be significant. However, in clinical practice, it is seen to be a rare occurrence, with reported resistance of 0-10.8% in adults (Sundvall et al. 2014; Bollestad et al. 2018; Ny et al. 2019; Kresken et al. 2022; Plambeck et al. 2022; Stoltidis-Claus et al. 2023), and around 4% in children (Thaulow et al. 2021). Urinary tract infections continue to have a favourable clinical response to mecillinam, and the frequency of mecillinam resistance in E. coli remains relatively low, despite its use over the years as the first choice for the UTIs treatment in most countries (Thulin et al. 2015; Nielsen et al. 2019). Thus, it has been of high interest to understand and describe resistance mechanisms and their different in vivo and in vitro manifestations.
In clinical isolates of E. coli, isolated from UTIs, inactivation of the cysB gene, which encodes the main regulator of cysteine biosynthesis, is the major cause of mecillinam resistance in clinical strains (Thulin et al. 2015). CysB mutations induce a gene regulatory response that alters the expression of more than 400 genes. These cysB mutants exhibit increased expression of genes involved in the regulation of several proteins, including PBP1B and LpoB. In cases when both are overexpressed, they act as a main resistance mechanism, reviving E. coli cells previously inhibited by mecillinam. However, the introduction of various reducing agents to these strains is shown to lead to the reversal of resistance and a drop in aforementioned protein levels. This provides an opportunity to externally modify the resistance and enhance treatment success (Thulin & Andersson, 2019). Mecillinam-resistant strains of E. coli with a cysB mutation, including both laboratory and clinical isolates, consistently exhibited greater susceptibility to mecillinam when cultivated in urine compared to laboratory medium. This indicates that mecillinam may still be a viable therapeutic choice for clinical isolates of E. coli that are classified as mecillinam-resistant in laboratory assessments, whether through disc diffusion or standardized MIC assays. The susceptibility level differed among urine samples collected from various individuals, showing a correlation with urine osmolality. Mecillinam had high efficacy against bacteria that were resistant in vitro, specifically in urine with low osmolality. Enhancing water consumption or employing diuretics to decrease urine osmolality alongside elevated cysteine intake may potentially enhance antibiotic susceptibility in resistant strains of E. coli, thereby improving treatment efficacy (Thulin et al. 2017). Mecillinam-resistant mutants are distinctive in that they developed through a single-step mutation, where a solitary genetic alteration could grant clinical resistance to mecillinam (Podnecky et al. 2018). But, as previously described, this is the first example exhibiting conditional resistance where a genetically stable antibiotic resistance can be phenotypically reverted to susceptibility by metabolites present in its surroundings, urine in this case.
The clinical strains displayed much higher levels of fitness and a wide variety of mutations, in comparison to the laboratory-isolated mutants. The majority of clinical mecillinam resistant isolates have a reduced rate of proliferation, hence decreasing the likelihood of their stable persistence within the bladder (Thulin et al. 2015). In general, mutants resistant to mecillinam display severely reduced growth rates, suggesting high costs of resistance (Podnecky et al. 2018). Therefore, it is likely that the same dosing regimen can effectively treat all clinical MDR isolates of E. coli, including those that showed resistance to mecillinam under laboratory settings (Zykov et al. 2020).
Apart from previously described mechanisms of resistance, a narrow-spectrum β-lactamase CTX-M-215 was identified in an E. coli clinical isolate in China in 2020. Exhibiting high-level resistance to mecillinam, CTX-M-215 differed from the previously known CTX-M-125, a CTX-M ESBL, indicating an in vivo evolution of these mutations. Fortunately, this strain was sensitive to cefotaxime (Yin et al. 2020). Therefore, despite the remarkable antibacterial characteristics exhibited by mecillinam, the uncontrolled utilization of this antibiotic carries significant risks and may result in potentially life-threatening outcomes.
4. Conclusion
The findings of the reviewed literature support the recommendation that mecillinam should be used as the first-line option for empirical treatment of uncomplicated lower UTIs caused by E. coli. Mecillinam has shown a low increase in the odds for selection of resistance, as well as good susceptibility in multidrug-resistant E. coli isolates, that persists over the years. These antimicrobial properties indicate that mecillinam is a favourable antibiotic of choice. An assessment of antibiotic resistance in E. coli isolates in Serbia is necessary, and the findings should be taken into account when updating the UTI treatment guidelines in Serbia.
Funding: This research received no external funding.
Informed Consent Statement: Not applicable.
Data Availability Statement: Not applicable.
Acknowledgments: None.
Conflicts of Interest: The authors declare no conflicts of interest.
References
- Bollestad, M., Vik, I., Grude, N., Blix, H. S., Brekke, H., & Lindbaek, M. (2018). Bacteriology in uncomplicated urinary tract infections in Norwegian general practice from 2001-2015. BJGP Open, 1(4), bjgpopen17X101145. https://doi.org/10.3399/bjgpopen17X101145
- Chen, Y. T., Ahmad Murad, K., Ng, L. S., Seah, J. T., Park, J. J., & Tan, T. Y. (2016). In Vitro Efficacy of Six Alternative Antibiotics against Multidrug Resistant Escherichia coli and Klebsiella pneumoniae from Urinary Tract Infections. Annals of the Academy of Medicine, Singapore, 45(6), 245–250.
- Cho, H., Uehara, T., & Bernhardt, T. G. (2014). Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery. Cell, 159(6), 1300–1311. https://doi.org/10.1016/j.cell.2014.11.017
- Delhaye, A., Collet, J.-F., & Laloux, G. (2016). Fine-Tuning of the Cpx Envelope Stress Response Is Required for Cell Wall Homeostasis in Escherichia coli. mBio, 7(1), e00047-00016. https://doi.org/10.1128/mBio.00047-16
- Djordjević, Z., Folić, M., Ninković, V., Vasiljević, D., & Janković, S. (2019). Antimicrobial susceptibility among urinary Escherichia coli isolates from female outpatients: Age-related differences. Central European Journal of Public Health, 27(3), 245–250. https://doi.org/10.21101/cejph.a4833
- EAU Guidelines. (2023). Edn. Presented at the EAU Annual Congress Milan, Italy 2023.
- Emeraud, C., Godmer, A., Girlich, D., Vanparis, O., Mahamdi, F., Creton, E., Jousset, A. B., Naas, T., Bonnin, R. A., & Dortet, L. (2022). Activity of mecillinam against carbapenem-resistant Enterobacterales. The Journal of Antimicrobial Chemotherapy, 77(10), 2835–2839. https://doi.org/10.1093/jac/dkac226
- Fagan, M., Lindbæk, M., Grude, N., Reiso, H., Romøren, M., Skaare, D., & Berild, D. (2015). Antibiotic resistance patterns of bacteria causing urinary tract infections in the elderly living in nursing homes versus the elderly living at home: An observational study. BMC Geriatrics, 15, 98. https://doi.org/10.1186/s12877-015-0097-x
- Farfour, E., Dortet, L., Guillard, T., Chatelain, N., Poisson, A., Mizrahi, A., Fournier, D., Bonnin, R. A., Degand, N., Morand, P., Janvier, F., Fihman, V., Corvec, S., Broutin, L., Le Brun, C., Yin, N., Héry-Arnaud, G., Grillon, A., Bille, E., … On Behalf Of The Gmc Study Group, null. (2022). Antimicrobial Resistance in Enterobacterales Recovered from Urinary Tract Infections in France. Pathogens (Basel, Switzerland), 11(3), 356. https://doi.org/10.3390/pathogens11030356
- Fatsis-Kavalopoulos, N., Roemhild, R., Tang, P.-C., Kreuger, J., & Andersson, D. I. (2020). CombiANT: Antibiotic interaction testing made easy. PLoS Biology, 18(9), e3000856. https://doi.org/10.1371/journal.pbio.3000856
- Fuchs, F., Ahmadzada, A., Plambeck, L., Wille, T., & Hamprecht, A. (2020). Susceptibility of Clinical Enterobacterales Isolates With Common and Rare Carbapenemases to Mecillinam. Frontiers in Microbiology, 11, 627267. https://doi.org/10.3389/fmicb.2020.627267
- Fuchs, F., & Hamprecht, A. (2019). Results from a Prospective In Vitro Study on the Mecillinam (Amdinocillin) Susceptibility of Enterobacterales. Antimicrobial Agents and Chemotherapy, 63(4), e02402-18. https://doi.org/10.1128/AAC.02402-18
- Gajic, I., Jovicevic, M., Popadic, V., Trudic, A., Kabic, J., Kekic, D., Ilic, A., Klasnja, S., Hadnadjev, M., Popadic, D. J., Andrijevic, A., Prokic, A., Tomasevic, R., Ranin, L., Todorovic, Z., Zdravkovic, M., & Opavski, N. (2023). The emergence of multi-drug-resistant bacteria causing healthcare-associated infections in COVID-19 patients: A retrospective multi-centre study. Journal of Hospital Infection, 137, 1–7. https://doi.org/10.1016/j.jhin.2023.04.013
- Hansen, B. Å., Grude, N., Lindbæk, M., & Stenstad, T. (2022). The efficacy of pivmecillinam in oral step-down treatment in hospitalised patients with E. coli bacteremic urinary tract infection; a single-arm, uncontrolled treatment study. BMC Infectious Diseases, 22(1), 478. https://doi.org/10.1186/s12879-022-07463-7
- Hickman, R. A., Hughes, D., Cars, T., Malmberg, C., & Cars, O. (2014). Cell-wall-inhibiting antibiotic combinations with activity against multidrug-resistant Klebsiella pneumoniae and Escherichia coli. Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases, 20(4), O267-273. https://doi.org/10.1111/1469-0691.12374
- Jensen, M. L. V., Siersma, V., Søes, L. M., Nicolaisdottir, D., Bjerrum, L., & Holzknecht, B. J. (2022). Prior Antibiotic Use Increases Risk of Urinary Tract Infections Caused by Resistant Escherichia coli among Elderly in Primary Care: A Case-Control Study. Antibiotics (Basel, Switzerland), 11(10), 1382. https://doi.org/10.3390/antibiotics11101382
- Kahlmeter, G., Åhman, J., & Matuschek, E. (2015). Antimicrobial Resistance of Escherichia coli Causing Uncomplicated Urinary Tract Infections: A European Update for 2014 and Comparison with 2000 and 2008. Infectious Diseases and Therapy, 4(4), 417–423. https://doi.org/10.1007/s40121-015-0095-5
- Kocaoglu, O., & Carlson, E. E. (2015). Profiling of β-lactam selectivity for penicillin-binding proteins in Escherichia coli strain DC2. Antimicrobial Agents and Chemotherapy, 59(5), 2785–2790. https://doi.org/10.1128/AAC.04552-14
- Kresken, M., Pfeifer, Y., Wagenlehner, F., Werner, G., Wohlfarth, E., & Of The Paul Ehrlich Society For Infection Therapy, O. B. O. S. G. ‘Antimicrobial R. (2022). Resistance to Mecillinam and Nine Other Antibiotics for Oral Use in Escherichia coli Isolated from Urine Specimens of Primary Care Patients in Germany, 2019/20. Antibiotics, 11(6), 751. https://doi.org/10.3390/antibiotics11060751
- List, K. K., Kolpen, M., Kragh, K. N., Charbon, G., Radmer, S., Hansen, F., Løbner-Olesen, A., Frimodt-Møller, N., & Hertz, F. B. (2022). Synergy between Mecillinam and Ceftazidime/Avibactam or Avibactam against Multi-Drug-Resistant Carbapenemase-Producing Escherichia coli and Klebsiella pneumoniae. Antibiotics, 11(10), 1280. https://doi.org/10.3390/antibiotics11101280
- Ministarstvo Zdravlja Republike Srbije. (2004). Infekcije urinarnog trakta; Nacionalni Vodic Za Lekare u Primarnoj Zdravstvenoj Zastiti.
- Montelin, H., Forsman, K.-J., & Tängdén, T. (2019). Retrospective evaluation of nitrofurantoin and pivmecillinam for the treatment of lower urinary tract infections in men. PloS One, 14(1), e0211098. https://doi.org/10.1371/journal.pone.0211098
- Nielsen, K. L., Hansen, K. H., Nielsen, J. B., Knudsen, J. D., Schønning, K., Frimodt-Møller, N., Hertz, F. B., & Jansåker, F. (2019). Mutational change of CTX-M-15 to CTX-M-127 resulting in mecillinam resistant Escherichia coli during pivmecillinam treatment of a patient. Microbiology Open, 8(12), e941. https://doi.org/10.1002/mbo3.941
- Ny, S., Edquist, P., Dumpis, U., Gröndahl-Yli-Hannuksela, K., Hermes, J., Kling, A.-M., Klingeberg, A., Kozlov, R., Källman, O., Lis, D. O., Pomorska-Wesołowska, M., Saule, M., Wisell, K. T., Vuopio, J., Palagin, I., & NoDARS UTIStudy Group. (2019). Antimicrobial resistance of Escherichia coli isolates from outpatient urinary tract infections in women in six European countries including Russia. Journal of Global Antimicrobial Resistance, 17, 25–34. https://doi.org/10.1016/j.jgar.2018.11.004
- O’Kelly, F., Kavanagh, S., Manecksha, R., Thornhill, J., & Fennell, J. P. (2016). Characteristics of gram-negative urinary tract infections caused by extended spectrum beta lactamases: Pivmecillinam as a treatment option within South Dublin, Ireland. BMC Infectious Diseases, 16(1), 620. https://doi.org/10.1186/s12879-016-1797-3
- Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, n71. https://doi.org/10.1136/bmj.n71
- Plambeck, L., Fuchs, F., Sattler, J., & Hamprecht, A. (2022). In vitro activity of mecillinam, temocillin and nitroxoline against MDR Enterobacterales. JAC-Antimicrobial Resistance, 4(3), dlac059. https://doi.org/10.1093/jacamr/dlac059
- Podnecky, N. L., Fredheim, E. G. A., Kloos, J., Sørum, V., Primicerio, R., Roberts, A. P., Rozen, D. E., Samuelsen, Ø., & Johnsen, P. J. (2018). Conserved collateral antibiotic susceptibility networks in diverse clinical strains of Escherichia coli. Nature Communications, 9(1), 3673. https://doi.org/10.1038/s41467-018-06143-y
- Priyadharshana, U., Piyasiri, L. B., & Wijesinghe, C. (2019). Prevalence, antibiotic sensitivity pattern and genetic analysis of extended spectrum beta lactamase producing Escherichia coli and Klebsiella spp among patients with community acquired urinary tract infection in Galle district, Sri Lanka. The Ceylon Medical Journal, 64(4), 140–145. https://doi.org/10.4038/cmj.v64i4.8990
- Rosenkilde, C. E. H., Munck, C., Porse, A., Linkevicius, M., Andersson, D. I., & Sommer, M. O. A. (2019). Collateral sensitivity constrains resistance evolution of the CTX-M-15 β-lactamase. Nature Communications, 10(1), 618. https://doi.org/10.1038/s41467-019-08529-y
- Samuelsen, Ø., Overballe-Petersen, S., Bjørnholt, J. V., Brisse, S., Doumith, M., Woodford, N., Hopkins, K. L., Aasnæs, B., Haldorsen, B., Sundsfjord, A., & Norwegian Study Group on CPE. (2017). Molecular and epidemiological characterization of carbapenemase-producing Enterobacteriaceae in Norway, 2007 to 2014. PloS One, 12(11), e0187832. https://doi.org/10.1371/journal.pone.0187832
- Stoltidis-Claus, C., Rosenberger, K. D., Mandraka, F., Quante, X., Gielen, J., Hoffmann, D., Wisplinghoff, H., & Jazmati, N. (2023). Antimicrobial resistance of clinical Enterobacterales isolates from urine samples, Germany, 2016 to 2021. Euro Surveillance: Bulletin Europeen Sur Les Maladies Transmissibles = European Communicable Disease Bulletin, 28(19), 2200568. https://doi.org/10.2807/1560-7917.ES.2023.28.19.2200568
- Strand, L., Jenkins, A., Henriksen, I. H., Allum, A. G., Grude, N., & Kristiansen, B. E. (2014). High levels of multiresistance in quinolone resistant urinary tract isolates of Escherichia coli from Norway; a non clonal phenomen? BMC Research Notes, 7, 376. https://doi.org/10.1186/1756-0500-7-376
- Sundvall, P.-D., Elm, M., Gunnarsson, R., Mölstad, S., Rodhe, N., Jonsson, L., & Ulleryd, P. (2014). Antimicrobial resistance in urinary pathogens among Swedish nursing home residents remains low: A cross-sectional study comparing antimicrobial resistance from 2003 to 2012. BMC Geriatrics, 14, 30. https://doi.org/10.1186/1471-2318-14-30
- Thaulow, C. M., Lindemann, P. C., & Klingenberg, C. (2021). Antibiotic resistance in paediatric UTIs in Norway. Tidsskrift for Den Norske Laegeforening: Tidsskrift for Praktisk Medicin, Ny Raekke, 141(10). https://doi.org/10.4045/tidsskr.20.0889
- Thulin, E., & Andersson, D. I. (2019). Upregulation of PBP1B and LpoB in cysB Mutants Confers Mecillinam (Amdinocillin) Resistance in Escherichia coli. Antimicrobial Agents and Chemotherapy, 63(10), e00612-19. https://doi.org/10.1128/AAC.00612-19
- Thulin, E., Sundqvist, M., & Andersson, D. I. (2015). Amdinocillin (Mecillinam) resistance mutations in clinical isolates and laboratory-selected mutants of Escherichia coli. Antimicrobial Agents and Chemotherapy, 59(3), 1718–1727. https://doi.org/10.1128/AAC.04819-14
- Thulin, E., Thulin, M., & Andersson, D. I. (2017). Reversion of High-level Mecillinam Resistance to Susceptibility in Escherichia coli during Growth in Urine. EBioMedicine, 23, 111–118. https://doi.org/10.1016/j.ebiom.2017.08.021
- Tutone, M., Bjerklund Johansen, T. E., Cai, T., Mushtaq, S., & Livermore, D. M. (2022). Susceptibility and Resistance to Fosfomycin and other antimicrobial agents among pathogens causing lower urinary tract infections: Findings of the SURF study. International Journal of Antimicrobial Agents, 59(5), 106574. https://doi.org/10.1016/j.ijantimicag.2022.106574
- Yin, M., Hu, G., Shen, Z., Fang, C., Zhang, X., Li, D., Doi, Y., Zhang, Y., Wang, M., & Guo, Q. (2020). In Vivo Evolution of CTX-M-215, a Novel Narrow-Spectrum β-Lactamase in an Escherichia coli Clinical Isolate Conferring Resistance to Mecillinam. Antimicrobial Agents and Chemotherapy, 64(11), e00562-20. https://doi.org/10.1128/AAC.00562-20
- Zykov, I. N., Frimodt-Møller, N., Småbrekke, L., Sundsfjord, A., & Samuelsen, Ø. (2020). Efficacy of mecillinam against clinical multidrug-resistant Escherichia coli in a murine urinary tract infection model. International Journal of Antimicrobial Agents, 55(2), 105851. https://doi.org/10.1016/j.ijantimicag.2019.11.008
- Нови национални водич за рационалну употребу антибиотика. (2018). https://www.zdravlje.gov.rs/tekst/335899/novi-nacionalni-vodic-za-racionalnu-upotrebu-antibiotika-.phpref/sajt
