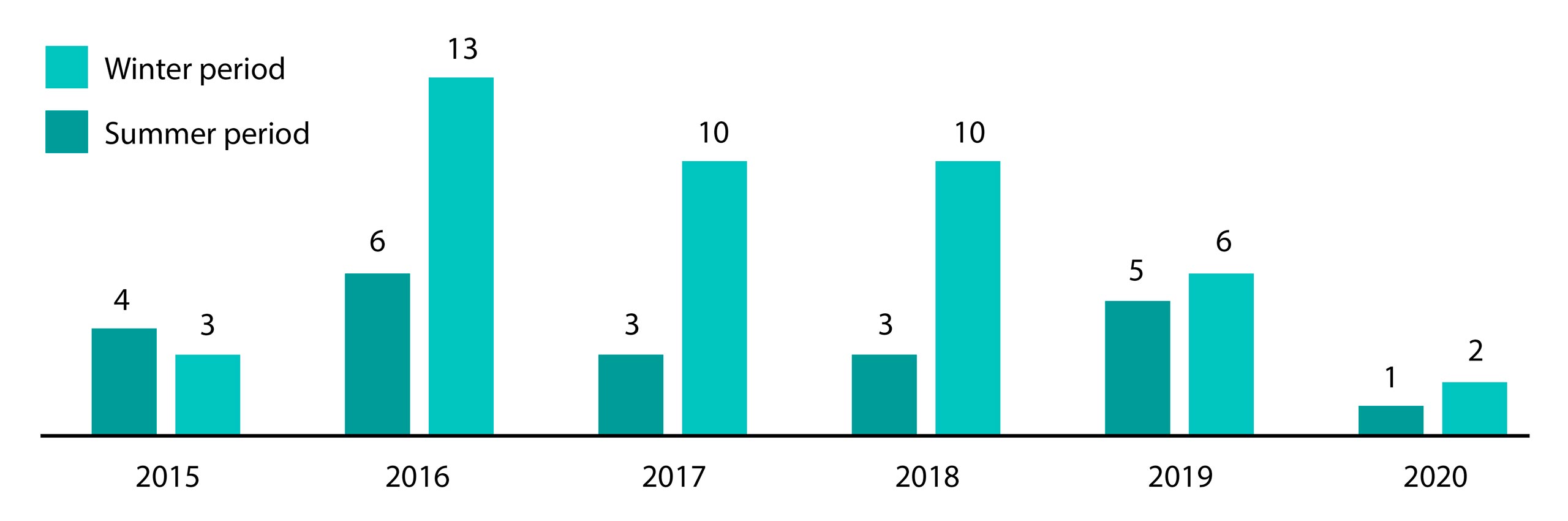1. Introduction
Streptococcus pneumoniae (S. pneumoniae) is a Gram-positive bacterium with characteristic lancet-shaped cocci morphology, often found in pairs (diplococci) or arranged in small chains. Pneumococcus is one of the most important human pathogens. S. pneumoniae belongs to the alpha-hemolytic streptococci. A positive optochin test is what distinguishes it from other streptococci typically found in the upper respiratory tract flora. The key virulence factor of S. pneumoniae is its capsule, which also possesses antigenic properties. Besides the capsule, pneumococcus possesses a range of additional virulence factors such as pneumolysin, autolysin, pneumococcal surface protein A, CbpA (choline-binding protein A), PsaA (pneumococcal surface adhesin A), pili, neuraminidase, bacteriocin, IgA protease and other which significantly contribute to increasing the invasive potential of pneumococcus (Savic et al. 2019; Kalenic, 2013).
Pneumococcus primarily manifests clinically through notable conditions including otitis media, sinusitis, conjunctivitis, pneumonia, and the occurrence of invasive pneumococcal disease (IPD). These conditions arise when the pathogen breaches the normally sterile sites such as the bloodstream and cerebrospinal fluid, causing sepsis and meningitis [Popovic et al, 2018; Gajic et al, 2016) . Infection of the middle ear is very common in children, primarily attributed to anatomical predispositions and the higher prevalence of pneumococci within the child’s nasopharyngeal microbiome (Kalenic, 2013). Meningitis caused by S. pneumoniae has a more frequent mortality rate compared to other meningitis-causing bacteria. Additionally, 50% of patients afflicted with pneumococcal meningitis experience serious neurological manifestations (Savic et al. 2019).
Pneumococcal pneumonia is the most common community-acquired pneumonia. Pneumonia arises from the aspiration of pneumococcus, a bacterium normally found in the nasopharynx. After the S. pneumoniae reaches the alveoli, the alveolar macrophages attempt to engulf the bacteria, but the pneumococcal capsule prevents phagocytosis leading to the decay of macrophages. Subsequently, pneumococcus releases its enzymes, including pneumolysin, which acts as a cytotoxin and forms pores in the cells of the respiratory epithelium. By this point, inflammation has already begun, characterized by a significant presence of purulent exudate. Moreover, pneumococcal pneumonia is notable for its purulent nature and typically presents as lobar pneumonia, affecting an entire lobe of the lung (Kalenic, 2013). Inflammation can propagate directly from the lungs, where pneumonia is concentrated, to the pleura either through hematogenous or lymphogenous spread, resulting in pleurisy. Pleurisy is characterized by the presence of purulent exudate in the pleural cavity, known as empyema pleurae. Once the infection infiltrates the pleural cavity, it can easily enter the bloodstream, leading to bacteremia, a condition observed in 30% of patients. Pneumonia frequently occurs in children, but it is also prevalent among individuals with chronic lung diseases or in elderly patients who have previously experienced viral infections, which weaken local respiratory tract immunity. S. pneumoniae is most commonly isolated from various sources, including fluid aspirated from the middle ear, pleural punctures, blood, samples from the lower respiratory tract (such as sputum, bronchoalveolar lavage, and broncho-aspirate), and cerebrospinal fluid. The capsule plays a central role in the process of serotyping due to its significance as the primary virulence and antigenic factor. Serotypes are distinguished by the type and arrangement of sugars within the capsules. To date, a total of 97 serotypes of S. pneumoniae have been identified, with approximately ten of them being recognized as the most common causative agents of invasive pneumococcal diseases. Some of the most commonly isolated invasive serotypes were: 1, 5, 6A, 6B, 14, 19F, and 23F (Gajic et al, 2016; Milojevic et al, 2020). These serotypes are more likely to spread to typically sterile areas after the infection has progressed. Also, these serotypes exhibit resistance to a wide range of antibiotics, which adds complexity and diminishes the chances of successfully treating illnesses caused by them. The aims of the study were to identify the prevalence of the most common serotypes in invasive S. pneumoniae isolates among pneumonia patients, to assess their susceptibility to antimicrobial agents, and to establish the seasonal distribution patterns of pneumococcal isolates.
2. Methods and material
The retrospective study was carried out at the Department of Microbiological Diagnostics within the Institute for Pulmonary Diseases of Vojvodina in Sremska Kamenica, Serbia, from January 2015 to December 2020. A total of 66 isolates were incorporated into the study, all of which were obtained from blood and pleural fluid samples of pneumonia patients.
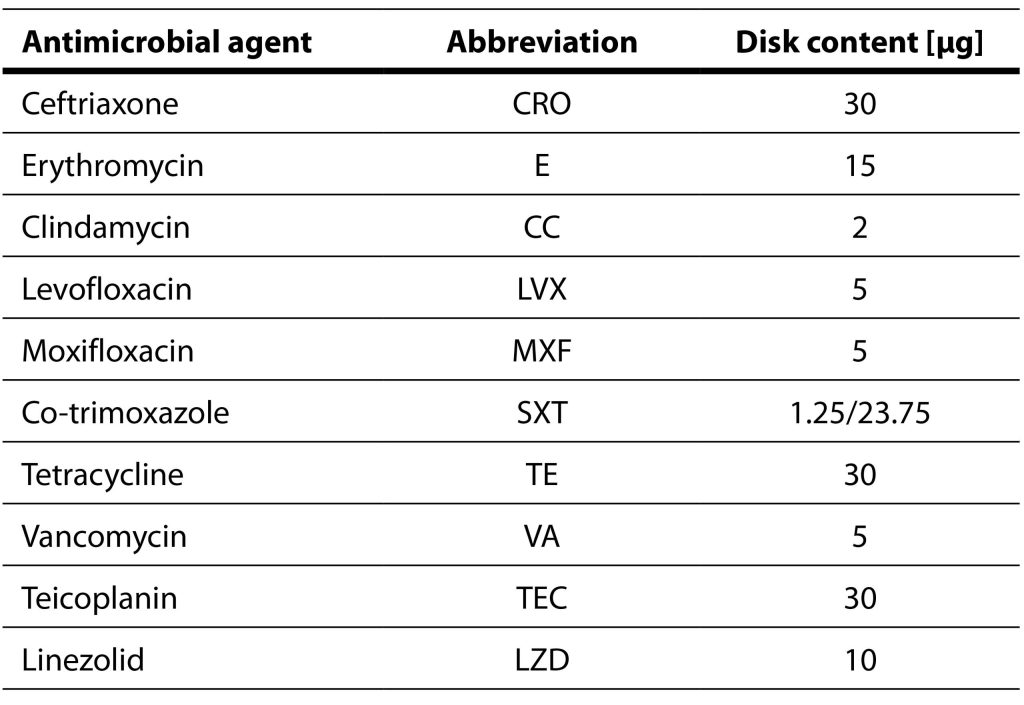
The blood cultures were incubated in an automated BacT/Alert system (BacT/Alert 3D, bioMérieux, France). Pleural fluid samples are cultured both in aerobic and anaerobic conditions, using standard cultivation techniques. Antimicrobial susceptibility testing was conducted for each S. pneumoniae isolate utilizing the disc-diffusion method (Table 1). As a control strain, Streptococcus pneumoniae ATCC 49619 was used. The interpretation of antimicrobial susceptibility testing was done according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommendations for the 2019 (EUCAST, 2019). To determine the minimal inhibitory concentrations for penicillin and cefotaxime, gradient tests were conducted using MIC Test Strip Penicillin G and MIC Test Strip Cefotaxime (Liofilchem, Rosetodegli Abruzzi, Italy).
Table 1. Antimicrobial agents tested using disc-diffusion method
The identification of isolates was confirmed by PCR detection of the lytA gene in the National Reference Laboratory for Streptococci in Belgrade (Gillespie, 1994). Serotyping was carried out using the Quelling reaction, employing serogroup and serotype antisera targeting the capsular polysaccharides (Lalitha, 1996).
3. Results
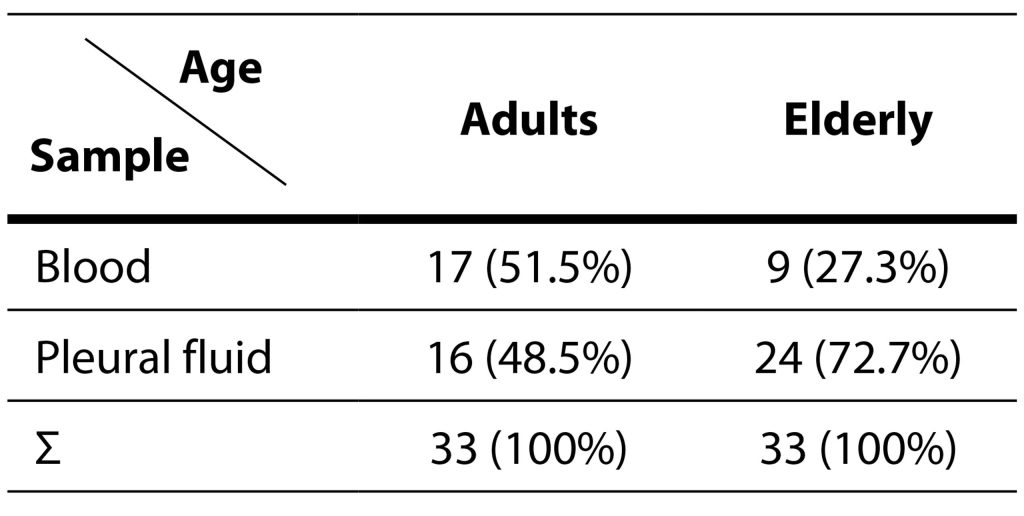
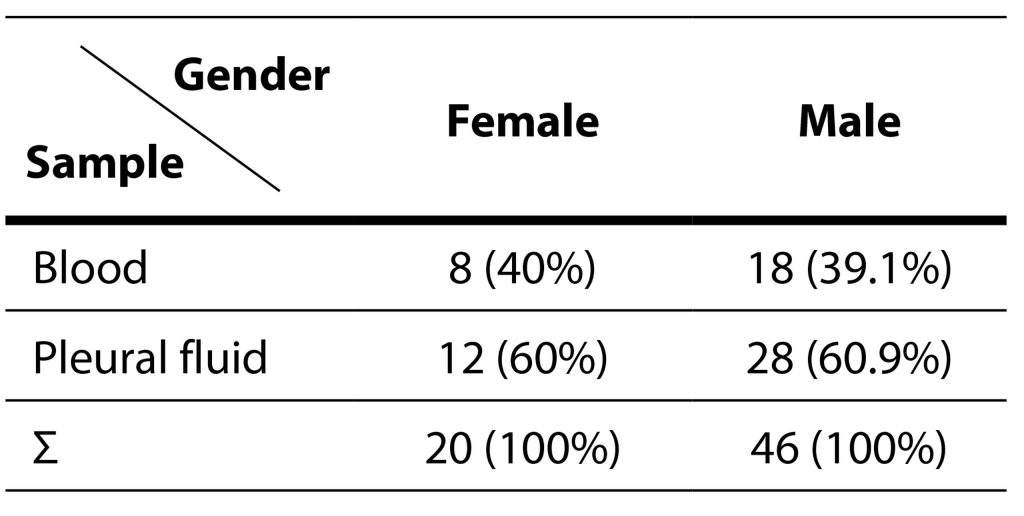
During the evaluation period, 66 isolates were obtained from the same number of patients. Among the examinees, 46 (69.7%) were males, while 20 (30.3%) were females. The isolation of pneumococci is statistically more significant in male patients in relation to female patients (χ2=5,32; p=0,02). The age range of the patients ranged from 21 to 87 years, demonstrating a homogenous distribution across different age groups. The patients were categorized into two groups: adults (those younger than 65 years) and the elderly (those aged 65 or older). Both groups comprised 33 patients each. Among the 46 male patients, 22 were classified as adults, and 24 as elderly. Of the 20 female patients, 11 fell into the adult category, while 9 were considered elderly. Detailed information about the relationship between the samples from which pneumococci were isolated based on age and gender can be found in Tables 2 and 3.
Table 2. Isolation of Streptococcus pneumoniae in various samples relative to age
Table 3. Isolation of Streptococcus pneumoniae in various samples relative to gender
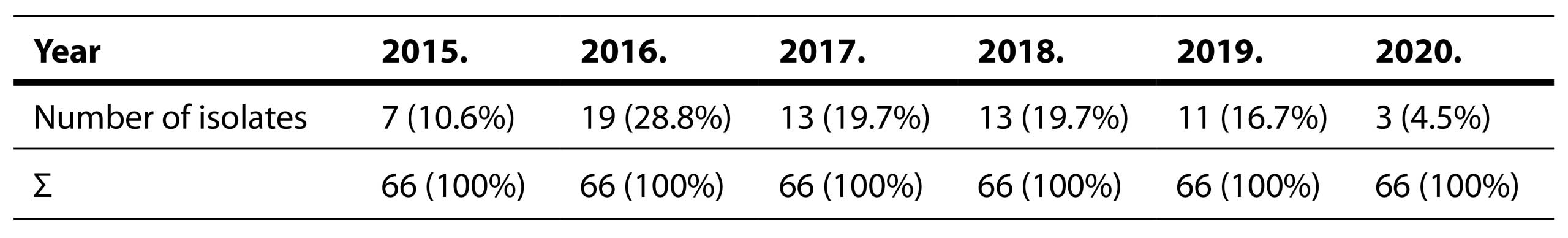
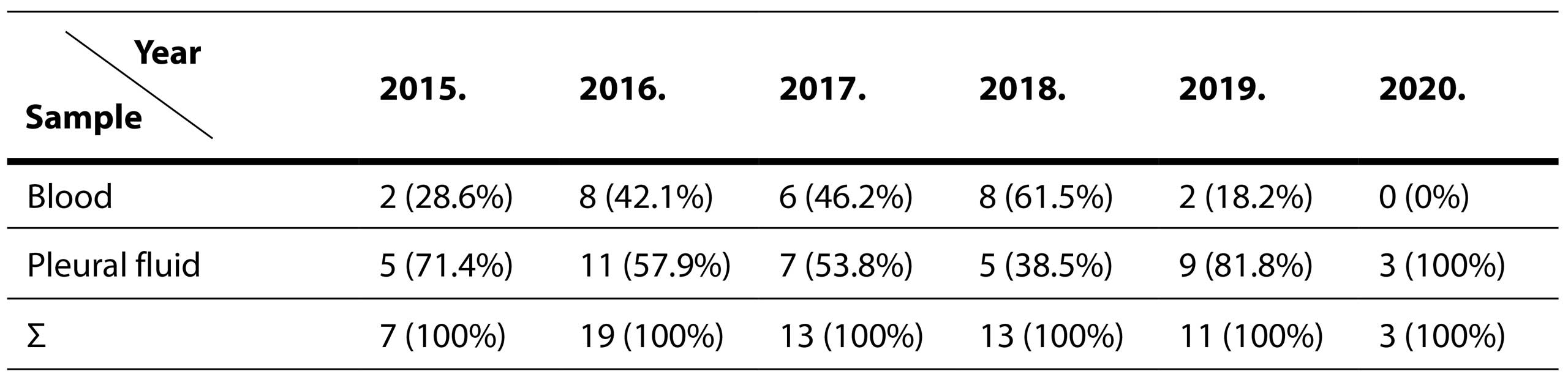
In Tables 4 and 5 Distribution Of Streptococcus pneumoniae Isolation By Years and Across Different Sample Types are shown.
Table 4. Distribution of Streptococcus pneumoniae isolation by years
Table 5. Distribution of Streptococcus pneumoniae isolation across different sample types
A total of 66 isolates of S. pneumoniae were obtained during the assessment period, with 40 (60.6%) of them isolated from pleural fluids and 26 from blood samples.
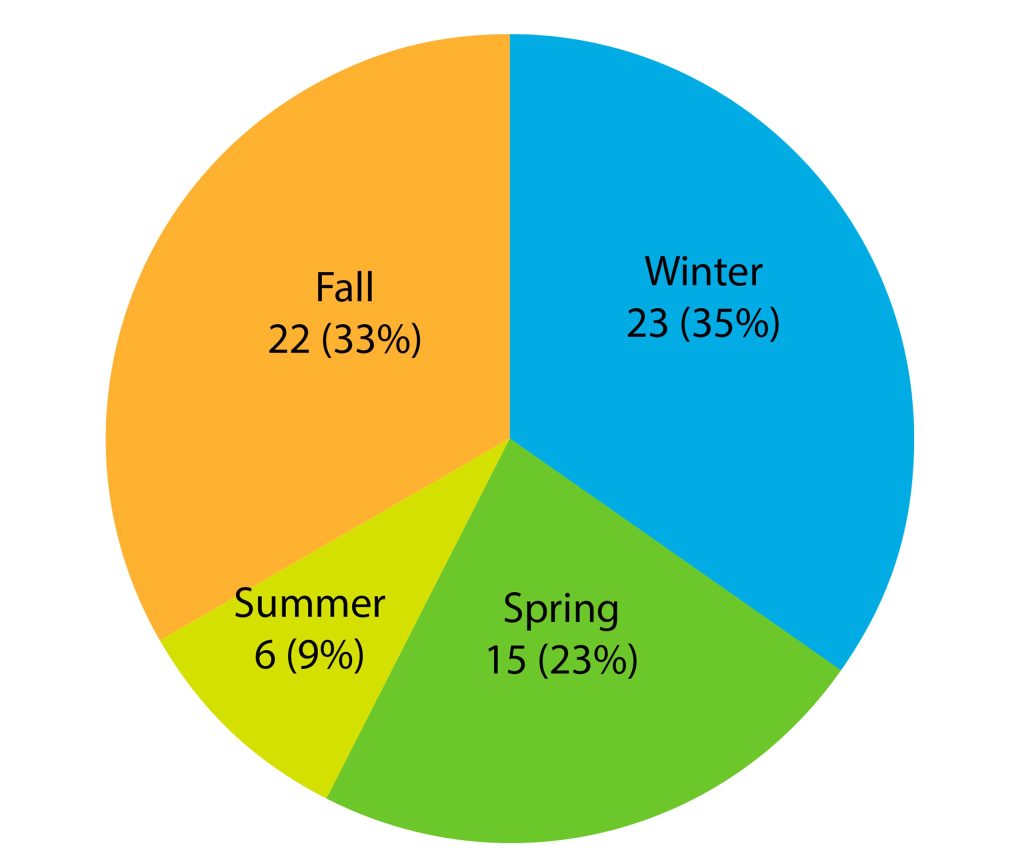
The assessment period was divided into seasons with the following start and end dates: Spring (March 21 – June 21), Summer (June 21 – September 23), Autumn (September 23 – December 21), and Winter (December 21 – March 21). This seasonal breakdown was used for grouping the strains. The distribution of isolates per season is illustrated in Graph 1.
Graph 1. Seasonal variation in Streptococcus pneumoniae isolation from 2015 to 2020
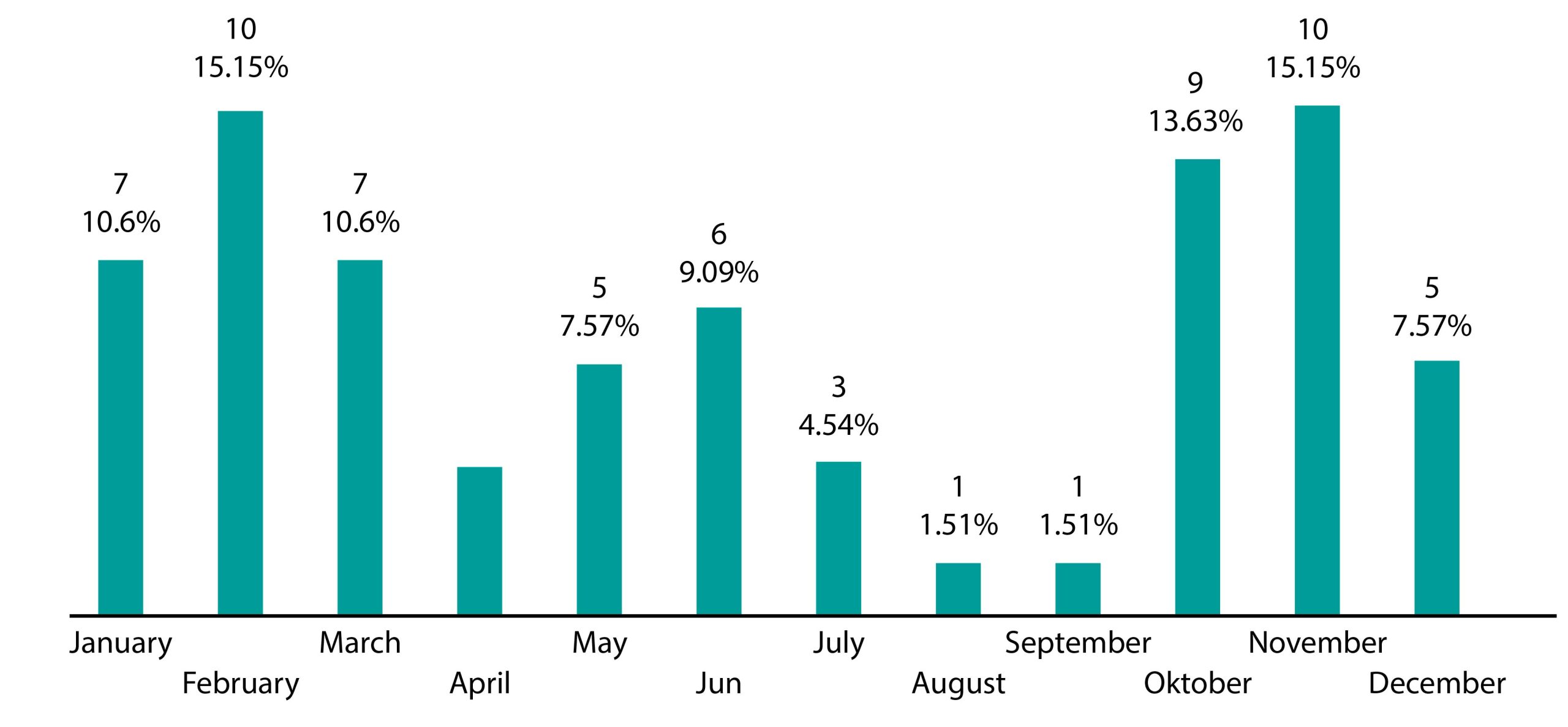
Graph 2 depicts the number of patients across the months. The year is divided into two distinct periods, namely summer and winter. The winter period encompasses autumn and winter, while the summer period includes spring and summer.
Graph 2. Variation in Streptococcus pneumoniae isolation by month
The isolation of pneumococci is more prevalent during the winter period (68.1%) compared to the summer period (31.8%), as demonstrated in Graph 3. It is important to note that there is no significant difference in isolations between the summer and winter periods.
Graph 3. Streptococcus pneumoniae isolation across summer and winter seasons from 2015 to 2020
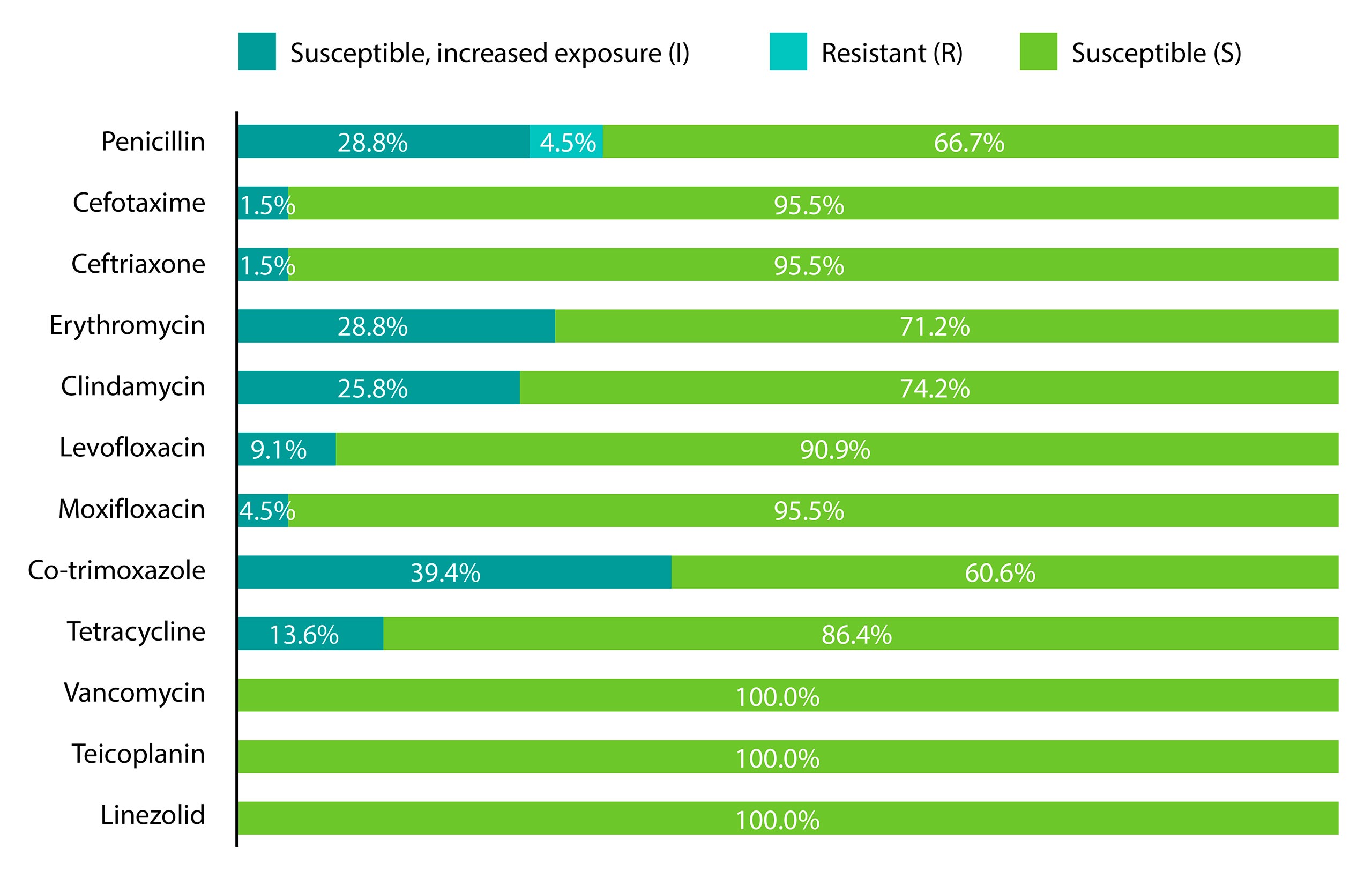
In the assessment period, the resistance percentages of pneumococci to various antibiotics were as follows: co-trimoxazole – 39.4%, erythromycin – 28.8%, clindamycin – 25.8%, tetracycline – 13.7%, levofloxacin – 9.1%, penicillin – 4.5%, moxifloxacin – 4.5%, cefotaxime – 1.5%, and ceftriaxone – 1.5% (Graph 4). Notably, isolates showed complete sensitivity to vancomycin, teicoplanin, and linezolid.
Graph 4. Antimicrobial susceptibility of Streptococcus pneumoniae isolates
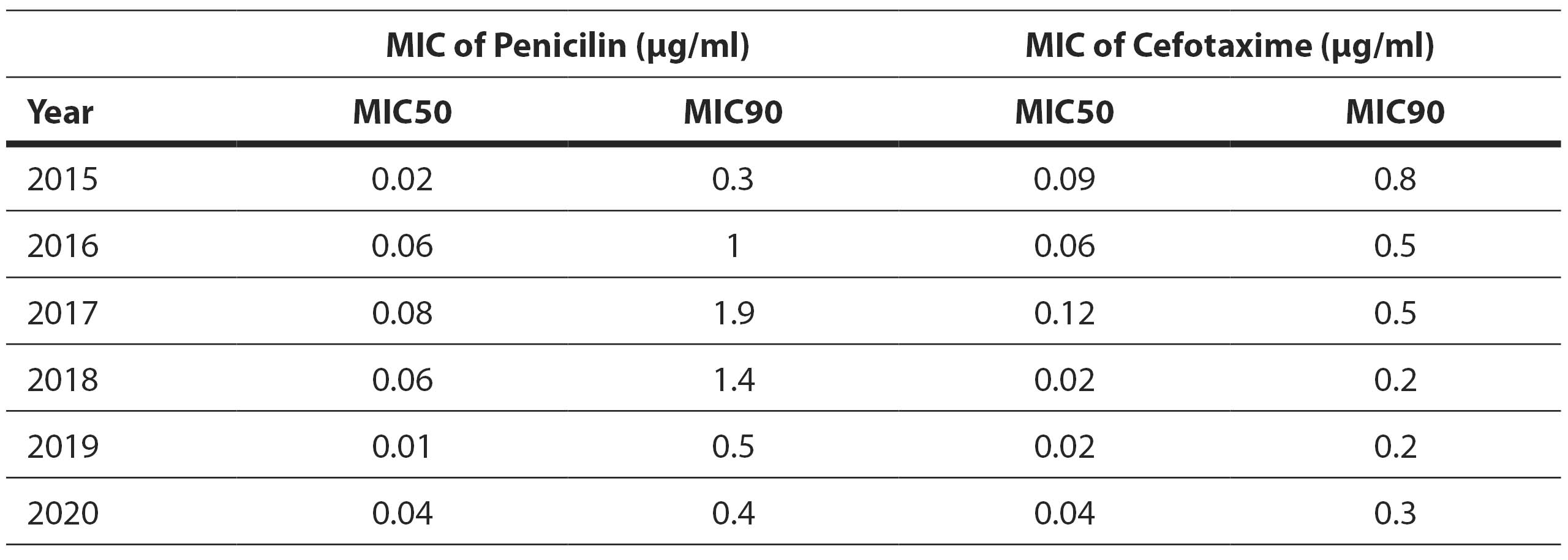
Furthermore, minimum inhibitory concentration tests were conducted for penicillin and cefotaxime. The MIC50 for penicillin was 0.06 μg/ml and the MIC90 was 1 μg/ml. The MIC50 for cefotaxime was 0.032 μg/ml and MIC90 was 0,5 μg/ml (Table 6).
Table 6. Minimum inhibitory concentration (MIC) distribution of penicillin and cephotaxime per year
MIC – minimum inhibitory concentration
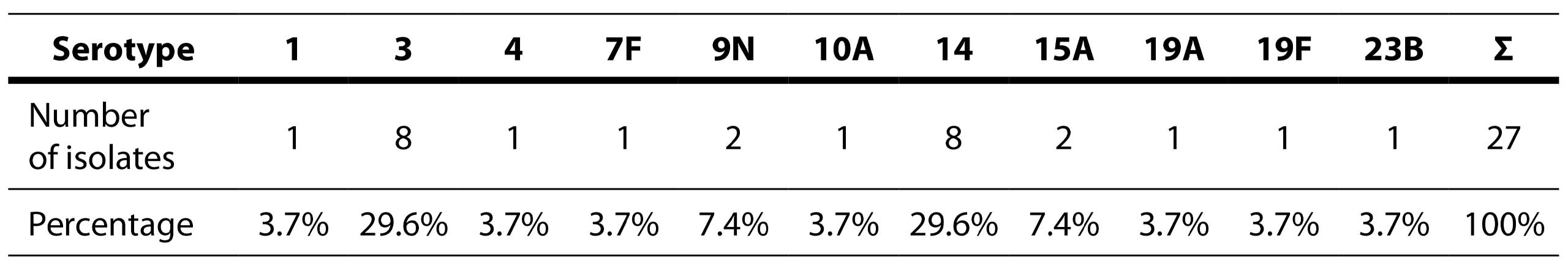
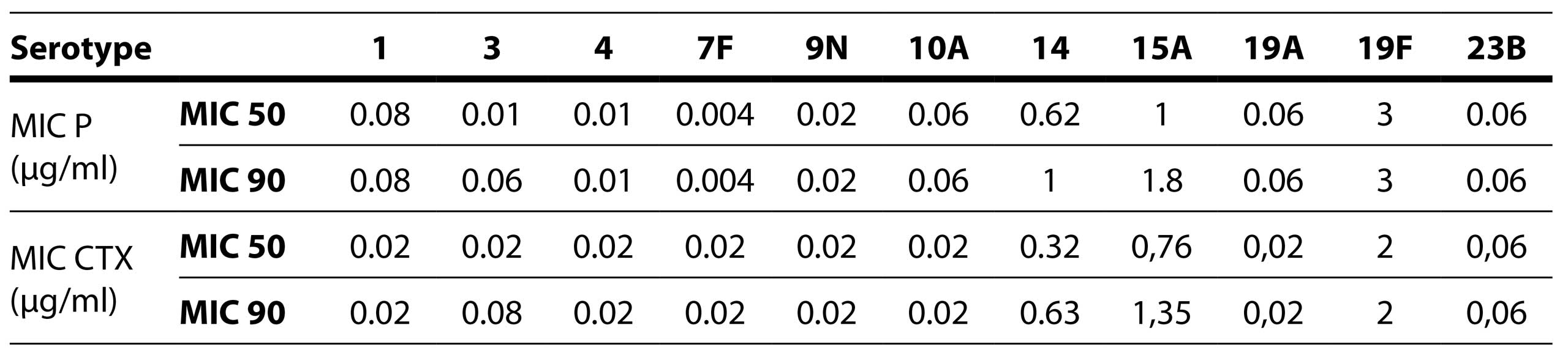
A total of 27 (40.9%) isolates were sent to the National Reference Laboratory for serotyping. The analysis revealed a total of 11 distinct serotypes: 1, 3, 4, 7F, 9N, 10A, 14, 15A, 19A, 19F, and 23B (Table 7).
Table 7. Distribution of detected serotypes
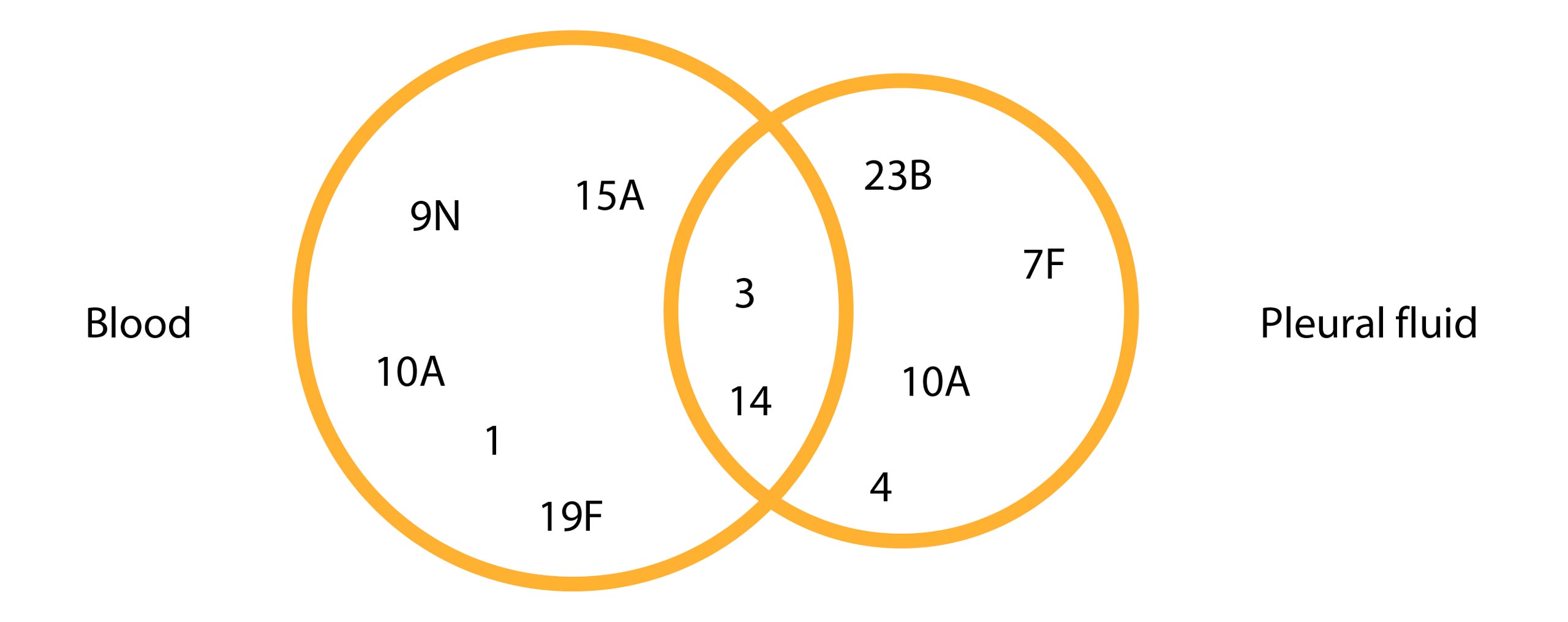
Among these, 11 serotypes were identified in pleural fluid, while 16 serotypes were isolated from blood samples (Figure 1). Remarkably, four of the 11 isolated serotypes (9N, 10A, 1, 7F) demonstrated sensitivity to all antibiotics tested. Only two out of the 11 serotypes were found to be multiresistant: 19F and 15A. Serotype 19F exhibited reduced susceptibility to penicillin, third-generation cephalosporins, macrolides, and co-trimoxazole, while serotype 15A showed reduced susceptibility to penicillin, macrolides, co-trimoxazole, and tetracycline.
Figure 1. Pneumococcal strain serotypes in relation to samples origin
Table 8. MIC values for penicillin and cefotaxime by serotypes
MIC – minimum inhibitory concentration
4. Discussion
Streptococcus pneumoniae is a common etiological agent responsible for respiratory infections in both pediatric and adult populations. It ranks as a leading causative factor of community-acquired pneumonia, as well as otitis media in children. Furthermore, it frequently gives rise to invasive pneumococcal diseases like sepsis and meningitis when it breaches typically sterile sites such as the bloodstream and cerebrospinal fluid. In the context of pneumococcal pneumonia, complications arise when pleural effusion occurs, facilitating the bacterium’s penetration into the bloodstream.
The incidence rate in 2018 for IPD was 6.4 cases per 100,000 individuals, according to the European Centre for Disease Prevention and Control. This rate is higher compared to the 4.8 cases per 100,000 recorded in 2014 (ECDC, 2018). Due to the frequent occurrence of pneumococcal diseases, vaccination is recommended to reduce the frequency of invasive pneumococcal diseases in older adults, immunocompromised individuals, and children. In children, vaccination aims to decrease the incidence of pneumococcal meningitis, while in older adults, it aims to prevent pneumonia and its associated complications. In Serbia, this vaccine was introduced into the mandatory active immunization schedule in 2016 (Petrovic et al. 2016). The treatment of pneumococcal diseases necessitates the administration of antibiotic therapy. The use of antimicrobial therapy as a form of selective pressure may lead to the development of antibiotic resistance to certain agents (Doern, 2001).
This study encompasses strains isolated from adult patients with complicated pneumonia. When pneumonia is clinically and radiologically diagnosed in a patient, the causative agent is typically sought through microbiological examination of sputum. For IPD, the sample may involve pleural fluid or blood.
In our study, 66 S. pneumoniae strains were isolated from blood samples and pleural fluids over a six-year period. Among the patients, 69.7% were male, and 30.3% were female. According to data from the European Union in the annual epidemiological report on IPD from 2016, pneumococcus was more frequently isolated in males than females (ECDC, 2018). Based on age, patients in our study were divided into two age groups: adults and elderly, specifically those younger than 65 years and those older than 65 years. This division was made because the incidence of IPD is expected to be higher in individuals older than 65 years due to their weakened immunity. In our study, the number of S. pneumoniae isolates in adult and elderly patients was equal, whereas according to data from the European Centre for Disease Prevention and Control, S. pneumoniae isolation is more common in patients over 65 years of age (ECDC, 2018).
In terms of the prevalence of S. pneumoniae isolates over the years, our study reveals similar percentages from year to year. A reduction in the number of S. pneumoniae isolates was observed in the year 2020, which may be attributed to changes in the healthcare institution’s operational protocols due to the pandemic caused by the SARS-CoV-2 virus. In our investigation of the seasonal distribution of pneumococcus-induced cases of pneumonia, a higher incidence was present during the winter compared to the summer months. This trend aligns with findings from Spain, where the percentages of pneumococcal infections by season were as follows: spring 17%, summer 14%, autumn 13%, and winter 21% (Cilloniz et al, 2017). In another study on the seasonal pneumonia distribution, it was demonstrated that pneumonia diagnosed during the winter is most commonly caused by pneumococcus, while in the summer, Legionella pneumophila was the most frequent bacterial pathogen responsible for pneumonia (Herrera-Lara et al, 2013).
In our study, the interpretation of antimicrobial susceptibility of strains was conducted in accordance with the recommendations of the EUCAST. Since 2019, there has been a change in the definition for the previous category of “intermediate susceptibility,” which is now classified as “susceptible with higher exposure,” potentially affecting the obtained results. Penicillin was the drug of choice for treating pneumococcal diseases until the first resistant strain emerged in Austria in 1967 (Hansman & Bullen, 1967). Due to the widespread use of penicillin, resistance has become highly prevalent. Penicillin-resistant pneumococcus (PRP) becomes resistant after a mutation in the penicillin-binding protein, which inhibits beta-lactam antibiotics from performing their function. In our study, 33.3% of isolates exhibited non- or decreased susceptibility to penicillin (resistant and susceptible with higher exposure phenotype). The percentages of pneumococcal resistance to penicillin are higher compared to studies conducted in 2009-2011 when the percentage of resistant strains was 20% (Gajic et al, 2016). A study from 2010-2012 also reported a lower resistance rate (10.1%) for macrolide-resistant pneumococcal strains (MRSP) isolated from adults (Hadnadjev et al, 2014).
In our study, the MIC50 for penicillin was 0.06 μg/ml and MIC90 was 1 μg/ml with values ranging from 0.002 to 3 μg/ml. Research by Hadnadjev and colleagues, who analyzed macrolide-resistant strains, showed a higher MIC value for penicillin, averaging 0.75 μg/ml, with values ranging from 0.025 to 3 μg/ml (Hadnadjev et al, 2014). The higher MIC value for penicillin in the aforementioned study can be attributed to a different sample, considering that isolates from pediatric patients, who have a higher resistance rate to penicillins, were included. Reduced susceptibility to penicillin was observed, which may result in either an increased dosage of the administered drug or the need for an alternative antibiotic.
Within the group of cephalosporins, sensitivity to cefotaxime and ceftriaxone, representing third-generation cephalosporins, was evaluated. A total of 4.5% of isolates exhibited resistance to cefotaxime, with a MIC50 value of 0.032 μg/ml and MIC90 value of 0.5 μg/ml. In a study on MRSP conducted during 2010-2012, 15% of isolates showed resistance to cefotaxime with a MIC value of 0.5 μg/ml, which is twice as high as our result (Hadnadjev et al, 2014).
Regarding macrolides, resistance was present in 28.8% of pneumococcal strains for erythromycin and 25.8% for clindamycin. Co-resistance to penicillin and erythromycin was found in 21.2% of isolates. In a study from 2009-2011, erythromycin resistance was observed in 36% of tested strains, and co-resistance to penicillin and erythromycin was present in 21% of isolates, which correlates with the results obtained in our study (Gajic et al, 2016). Co-resistance in patients with meningitis was slightly higher, at 28.5% (Milojevic et al, 2020). Co-resistance in a study of pre-vaccine serotypes was 36.4% (Petrovic et al, 2016). The high resistance to macrolides is due to their frequent use in the therapy of community-acquired pneumonia. Co-resistance to penicillins and macrolides is widespread (Klugman et Lonks, 2005).
In a study conducted from 2009-2011, all isolates were susceptible to moxifloxacin and levofloxacin. A significant increase in resistance has been noted in the past decade, with resistance rates in this study at 4.5% for moxifloxacin and 9% for levofloxacin, indicating a growing resistance to fluoroquinolones (Gajic et al, 2016).
All isolates were sensitive to vancomycin, as in the earlier retrospective studies (Gajic et al, 2016; Hadnadjev et al, 2014). Teicoplanin and linezolid are reserve antibiotics less commonly used in the treatment of pneumococcal diseases, which is why all isolates were susceptible to them.
Resistance to co-trimoxazole reached 39.4%, representing the highest recorded resistance percentage compared to all other antibiotics. Very similar results of 43% resistance to this antibiotic were documented in the work of author Gajić and colleagues (Gajic et al, 2016). In the MRSP study, co-resistance with co-trimoxasole was recorded at 74.3%. High resistance to this antibiotic is a consequence of its extensive historical use for treating pneumococcal diseases due to its optimal efficacy and low cost (Hadnadjev et al, 2014). In our study, 46.9% of isolates were susceptible to all antibiotics, which aligns with the 40% figure obtained in the research by author Gajić (Gajic et al, 2016).
The most commonly isolated serotypes out of 27 sent for serotyping were 3 (29.6%) and 14 (29.6%). Similar results were found among strains from the pre-vaccination period: 3 (20.9%), 19F (20%), and 14 (10.5%), with half of the resistant strains belonging to serotypes 14 and 19F (Gajic et al, 2016).
The high prevalence of serotype 3 in the adult population was also observed in patients with meningitis and a study on serotype distribution in the pre-vaccination period. This serotype is characterized by favorable antibiotic susceptibility, corresponding to the results obtained in this study, as only one of the serotype 3 isolates showed resistance to levofloxacin, while all others were susceptible to all antibiotics (Milojević et al, 2020; Petrovic et al, 2016)]. Among serotype 14 isolates, none of the isolates were multidrug-resistant. Co-resistance to penicillins and macrolides is frequent in this serotype based on studies from the pre-vaccination period (Gajic et al, 2016). Serotype 14 was marked as multidrug-resistant, exhibiting resistance to penicillin, macrolides, and co-trimoxazole (Gajic et al, 2016; Hadnadjev et al, 2014). Multidrug resistance in our study was observed in serotype 19F, which showed resistance to penicillins, macrolides, cephalosporins, and co-trimoxazole, in line with results from other studies (Gajic et al, 2016; Hadnadjev et al, 2014). Less frequently isolated serotypes included 15A (7.4%) and 9N (7.4%), while all other serotypes were isolated only once (3.7%). These serotypes were as follows: 1, 4, 7F, 10A, 19A, 19F, 23B. One of the two isolates of serotype 15A exhibited multidrug resistance, with resistance to macrolides, co-trimoxazole, and tetracycline. Serotypes 4, 19A, and 23B showed resistance to co-trimoxazole. Serotypes 1, 7F, 9N, 10A were susceptible to all antibiotics. In a study by author Gajić, serotypes 7F and 9N were susceptible to all antibiotics, while serotypes 1 and 4 were susceptible to only one antibiotic (Gajic et al, 2016).
Our study covers the pre-vaccination period. Pneumococcal vaccines are categorized by composition into polysaccharide vaccines containing capsular polysaccharides of specific serotypes and conjugate vaccines in which, to enhance immunity, capsular polysaccharides are linked to protein carriers (http://nrlstrep.rs/prevencija.html#loaded). Existing vaccines include the seven-valent PCV7 vaccine, covering serotypes 4, 6B, 9V, 14, 18C, 19F, 23F; the ten-valent PCV10 vaccine, covering serotypes 1, 4, 5, 7F, 6B, 9V, 14, 18C, 19F, 23B; the thirteen-valent PCV13 vaccine, covering serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 19A, 19F, 18C, 23F; and the PPSV23 vaccine, covering serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 33F (Popovic et al, 2018; CDC, 2020). PCV10 and PCV13 vaccines are recommended by the Centers for Disease Control and Prevention for healthy children under two years of age and children over two years with certain underlying conditions that make them more susceptible to IPD. PPSV23 is recommended for individuals over 65 years of age and adult smokers (Pneumococcal Vaccine Monograph for Professionals – Drugs.com).
The most substantial serotype coverage occurred with PPSV23, as expected, primarily because this vaccine contains the highest number of serotypes and is intended for older patients, the focus of this study. This contrasts with other vaccines intended for the pediatric population, which contain serotypes more frequently isolated in that demographic. In a study by author Milojević and colleagues, the serotype coverage of adult patients with meningitis to PPSV23 was 77.8%, consistent with our results [8]. The research by author Petrović and colleagues on serotype distribution showed a serotype coverage with PPSV23 of 85.2%, also consistent with our findings. The introduction of a vaccine leading to a reduction in IPD incidence indirectly results in decreased antibiotic resistance since the reduced disease incidence will lead to decreased antibiotic use (Petrović et al, 2016). One of the negative consequences of vaccine introduction is that, after some time, there will be a phenomenon of serotype replacement, where the isolation of vaccine serotypes in IPD patients will decrease, while non-vaccine serotypes will increase in prevalence (Hadnadjev et al, 2014).
5. Conclusion
In conclusion, this study revealed several important findings regarding pneumococcal pneumonia. Gender distribution demonstrated a higher prevalence of the condition in male patients compared to their female counterparts. Seasonal distribution showed a clear winter predominance of pneumococcal pneumonia. Antibiotic resistance was primarily observed against co-trimoxazole, penicillin, erythromycin, clindamycin, levofloxacin, and moxifloxacin, while isolates exhibited substantial susceptibility to third-generation cephalosporins (cefotaxime and ceftriaxone), tetracycline, vancomycin, teicoplanin, linezolid. The most frequently isolated serotypes were 3, 4, 15A, and 9A, with serotypes 19F and 15A displaying multidrug-resistant characteristics. These findings contribute to our understanding of pneumococcal pneumonia epidemiology and antibiotic susceptibility, which can inform clinical management and public health strategies for this respiratory infection.
Funding: This research was not funded.
Informed Consent Statement: Not applicable.
Data Availability Statement: Not applicable.
Conflicts of Interest: The author declares no conflicts of interest.
References
Centers of disease control and prevention (CDC): Pneumococcal Vaccination. [updated 2020 September 1; cited 2021 April 11]. Available from: https://www.cdc.gov/pneumococcal/vaccination.html
Centers for Disease Control and Prevention (CDC), Pneumococcal Disease. 2020 [updated 2020 September 1; cited 2021 February 20]. Available from: https://www.cdc.gov/pneumococcal/about/symptoms-complications.html
Cilloniz, C., Ewig. S., Gabarrus. A., Ferrer. M., Mensa. J., Torres. A. et al. (2017). Seasonality of pathogens causing community-acquired pneumonia. Respirology, 22(4):778-785.
Doern. GV. (2001). Antimicrobial use and the emergence of antimicrobial resistance with Streptococcus pneumoniae in the United States. Clin Infect Dis, 33(3):187-192.
Drugs.com. [updated 2020 June 5; cited 2021 April 11]. Available from: https://www.drugs.com/ppa/pneumococcal-polysaccharide-vaccine-23-valent.html
European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters. [Updated 2019 January 1; cited 2021 February 12] Version 9.0, 2019 Available from: http://www.eucast.org.
European center of disease control and prevention (ECDC). Invasive pneumococcal disease – Annual Epidemiological Report for 2018 [Homepage on the Internet]. [updated 2020 September 8; cited 2021 April 10]. Available from: https://www.ecdc.europa.eu/en/publications-data/invasive-pneumococcal-disease-annual-epidemiological-report-2018
Gajić. I., Mijač. V., Ranin. L., Anđelković. D., Radičević. M., Opavski. N. (2016). Invazivni sojevi Streptococcus pneumoniaeu Srbiji: osteljivost na antibiotike i serotipovi. Srp Arh Celok Lek, 141(1-2):48-53.
Gillespie, S. H., Ullman, C., Smith, M. D., & Emery, V. (1994). Detection of Streptococcus pneumoniae in sputum samples by PCR. Journal of clinical microbiology, 32(5), 1308–1311.
Hadnađev. M., Považan. A., Gajić. I., Mijač. V., Kurucin. T., Opavski. N. (2014). Phenotypes and genotypes of macrolide-resistant Streptoccocus pneumoniae in Serbia. Arch Biol Sci, 66(1)91-105.
Hansman. D., Bullen. MM. (1967). A resistant pneumococcus. Lancet, 1:264−265.
Herrera-Lara. S., Fernández-Fabrellas. E., Cervera-Juan. A., Blanquer-Olivas. R. (2013). Do seasonal changes and climate influence the etiology of community acquired pneumonia? Arch Bronconeumol, 49(4):140-145.
Kalenić. S. Streptokoki. U: Kalenić S, urednik. Medicinska mikrobiologiija. Zagreb: Medicinska naklada; 2013. p.125-39.
Klugman. KP., Lonks. JR. (2005). Hidden epidemic of macrolide-resistant pneumococci. Emerg Infect Dis, 11:802−807.
Lalitha, M. K., Pai, R., John, T. J., Thomas, K., Jesudason, M. V., Brahmadathan, K. N., Sridharan, G., & Steinhoff, M. C. (1996). Serotyping of Streptococcus pneumoniae by agglutination assays: a cost-effective technique for developing countries. Bulletin of the World Health Organization, 74(4), 387–390.
Milojević. S., Kekić. D., Gajić. I., Mijač. V., Opavski. N. (2020). Karakterizacija sojeva Streptococcus pneumoniae izolovanih kod pacijenata sa meningitisom pre uvođenja pneumokokne konjugovane vakcine u Srbiju. Med Podml, 71(2):39-45.
Nacionalna referentna laboratorija za streptokok [updated 2016 February 2; cited 2021 April 11]. Available from: http://nrlstrep.rs/prevencija.html#loaded
Petrović. V., Šeguljev. Z., Ristić. M., Đekić-Malbaša. J., Radosavljević. B., Medić. D., et al. (2016). Streptococcus Pneumonie serotype distribution in Vojvodina before the introduction of pneumococcal conjugate vaccines into the National Immunization Program. Srp Arh Celok Lek, 144(1-2):48-53.
Popović. S., Hadnađev. M., Gajić. I., Mijač. V., Kekić. D., Smitran. A. et al. (2018). Characterization of macrolide-resistant non-invasive pneumococci in the pre-vaccine era in Serbia. Acta Microbiol Immunol Hung, 65(4):477-88.
Vučković Opavski. N. Streptococcus i Enterococcus. U: Savić B, Mitrović S, Jovanović T, urednici. Medicinska mikrobiologiija. Beograd: Medicinski fakultet Univerziteta u Beogradu, CIBID; 2019. p.141-60.