1. Introduction
Herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) are highly prevalent human pathogens with global prevalence rates of approximately 67% (HSV-1) and 13% (HSV-2). It is estimated that in 2016, 3.7 billion people worldwide were seropositive for HSV-1, while nearly 500 million were seropositive for HSV-2 (Zhu and Viejo-Borbolla, 2021). Most individuals come into contact with HSV-1 early in life through orolabial mucosa (direct contact, saliva), whereas HSV-2 infections typically occur later, most often through sexual contact. Infection with one type of HSV usually induces type-specific immunity (Zhu and Viejo-Borbolla, 2021; Alareeki et al. 2022).
Following primary contact, the virus establishes infection in skin or mucosal cells while simultaneously accessing the nerves that innervate the site of infection. The virus then moves via retrograde axonal transport to enter the bodies of peripheral neurons (Cupic 2022; Connolly et al. 2022). HSV-1 and HSV-2 exhibit the ability to maintain lifelong latent infections at these sites. In the latent form, the genome is circular or episomal. During reactivation, gene expression and viral DNA replication lead to the formation of new viral particles that leave the cell and travel along axonal microtubules anterogradely to the nerve ending (Samies et al. 2020). Recurrent infections are common, with approximately four reactivations per year for genital HSV-2 infection and one reactivation per year for HSV-1. Infection can be transmitted from mother to newborn, increasing the risk of serious consequences (Connolly et al. 2022).
The structure of HSV-1 and HSV-2 includes a large, linear double-stranded DNA genome, protected by an icosahedral capsid and surrounded by a tegument layer, enclosed by an envelope with viral glycoproteins (Zhu and Viejo-Borbolla, 2021; Cupic 2022). The viral antireceptors are glycoproteins B (gB) and C (gC). They bind to glycosaminoglycans (GAG) on the cytoplasmic membrane, and then through interaction with gD and various receptors, they enable cell entry (Sadowski et al. 2021). These steps play a crucial role in initiating the process of viral entry into the cell and determine the tropism for specific cells and tissues (Zhu and Viejo-Borbolla, 2021). Within the cell, HSV-1 and HSV-2 undergo a carefully regulated replication process that includes the expression of specific genes during the lytic cycle (Ahmad et al. 2020). Manifestations of HSV-1 and HSV-2 infection can range from asymptomatic to mild or life-threatening. In most immunocompetent individuals, HSV-1 causes mild and self-limiting disease, unlike infections in immunocompromised patients or during pregnancy. Diseases caused by HSV-1 include vesicular lesions on the lips, genital herpes, herpetic stromal keratitis (HSK), iridocyclitis, eczema herpeticum, and systemic disease in neonates (Zhu and Viejo-Borbolla, 2021; Cupic 2022). In immunocompromised individuals and neonates, HSV-1 can cause severe diseases such as meningitis and encephalitis. Genital herpes (more often HSV-2) is transmitted from mother to child (vertical transmission) during passage through the infected birth canal, causing neonatal herpes, which most commonly presents with CNS symptoms (encephalitis) (Cupic 2022). Although the host immune response plays a key role in controlling HSV-1 and HSV-2 infections, the virus is equipped with various virulence factors that modulate and evade the immune response. Understanding the molecular mechanisms underlying the interaction between HSV-1, HSV-2, and the host is crucial for developing therapeutic approaches and prevention strategies (Connolly et al. 2020; Singh et al. 2019).
Antiviral therapy involves a group of nucleoside analogs (Whitley and Baines, 2018). Nucleoside analogs, such as acyclovir (ACV), valacyclovir, penciclovir, famciclovir, trifluridine, idoxuridine, vidarabine, sorivudine, brivudine, ganciclovir, and valganciclovir, act by inhibiting viral DNA polymerase activity. A prophylactic vaccine is not yet in routine use but is currently under investigation (Connolly et al. 2020). The aim of this study was to determine the seroprevalence of anti-HSV-1 and anti-HSV-2 IgG antibodies among medical students in Belgrade.
2. Materials and Methods
2.1. Patients and samples
The study included 100 medical students from whom serum samples were collected. From each participant, 5-10 ml of blood was taken. Sample processing was conducted in the Virology Laboratory at the Institute of Microbiology and Immunology, Faculty of Medicine, University of Belgrade. Each sample was centrifuged for 5-10 minutes at 2000 x g. After centrifugation, the separated sera were stored at -20°C until further analysis. Each participant completed a questionnaire and signed informed consent. The study was approved by the Ethics Committee of the Faculty of Medicine, University of Belgrade, number 17/I-39.
2.2. Serological testing
To determine the titers of anti-herpes simplex virus 1 and 2 antibodies (anti-HSV-1 and anti-HSV-2) of the IgG class, commercial ELISA kits (Euroimmun Medical Diagnostics, Lübeck, Germany) were used according to the manufacturer’s instructions. ELISA is a method based on an antigen-antibody reaction where one of the reagents (anti-human IgG antibodies) is labeled with an enzyme (peroxidase). The test comprises several phases and is based on the principle that each reagent is introduced sequentially into a well, incubated for a specified time, and then washed to remove unbound reagents. After the reaction between the antigen and the labeled antibody, an appropriate substrate is added, which, under the catalytic action of the enzyme, produces a colored product. The color is then detected colorimetrically, where the intensity of the signal (expressed as absorbance) is proportional to the amount of the analyte in the sample. According to the protocol, an IgG antibody titer <16 RU/ml indicates a negative result, ≥16 and <22 RU/ml indicates a borderline positive result, and ≥22 RU/ml indicates a positive result.
2.3. Statistical analysis
Categorical data were presented as absolute and relative numbers in percentage form. Numerical data were presented as mean with standard deviation or median with range, depending on the data distribution. Normality of distribution was evaluated using mathematical (coefficient of variation, Shapiro-Wilk test) and graphical (histogram and box plot) methods. Independent groups were compared using the chi-square test or Fisher’s exact test, if conditions for the former were not met. Comparison of samples based on numerical data was performed using either the Student’s t-test or the Mann-Whitney U test, depending on data distribution. All statistical methods were considered significant at the 0.05 level. Statistical analysis was performed using IBM SPSS software, version 21.
3. Results
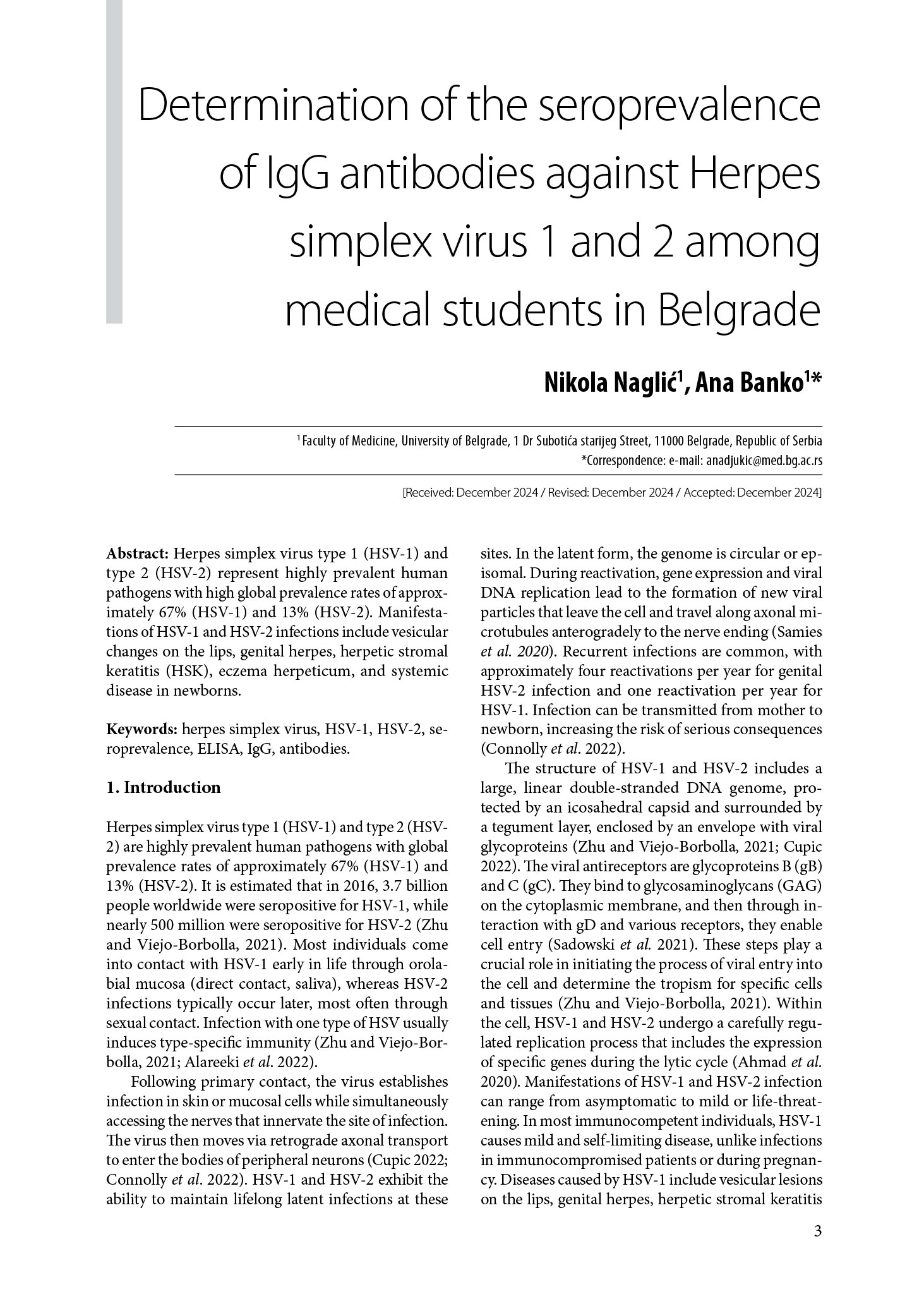
This cross-sectional study included a total of 100 medical students from the Faculty of Medicine, University of Belgrade, with an average age of 22.1±1.8 years (Figure 1), ranging from 19 to 28 years. The gender ratio was 1:3 in favor of females, with 76% female and 24% male participants. Most students were in their second or third year, followed by sixth, fourth, and fifth years.
Figure 1. Graphical representation of the age of participants included in the study (19-28).
Based on the completed questionnaires, most students attended kindergarten (84%) in urban areas (91%). Herpes labialis was reported by 27% of students, with 24 of them having had it only once, and three multiple times. No student reported having manifestations of genital herpes. The average titer of anti-HSV-1 IgG antibodies among participants was 97.3±38.7, while for anti-HSV-2 IgG antibodies it was 19.4±1.8.
3.1. Results by gender differences
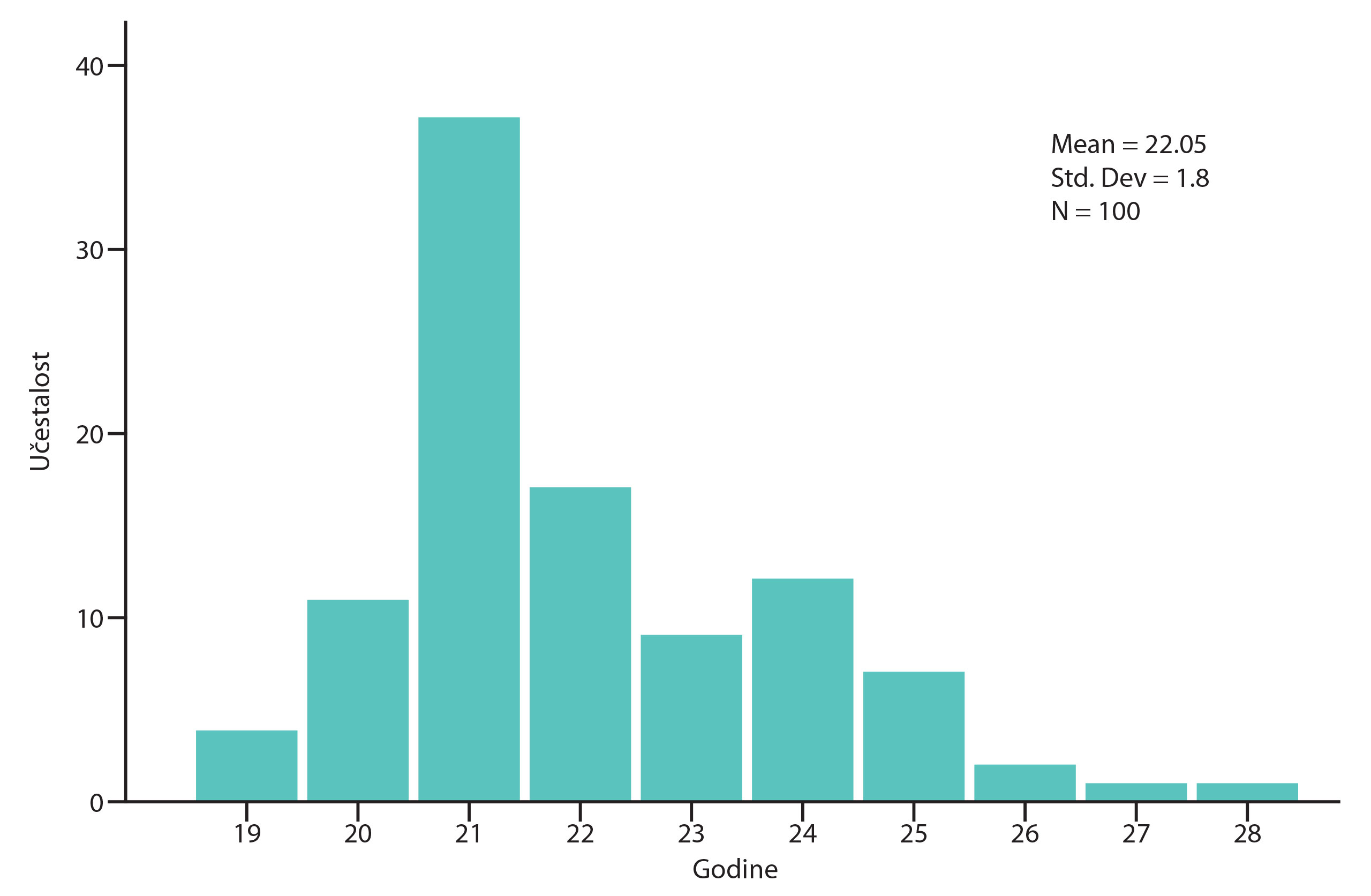
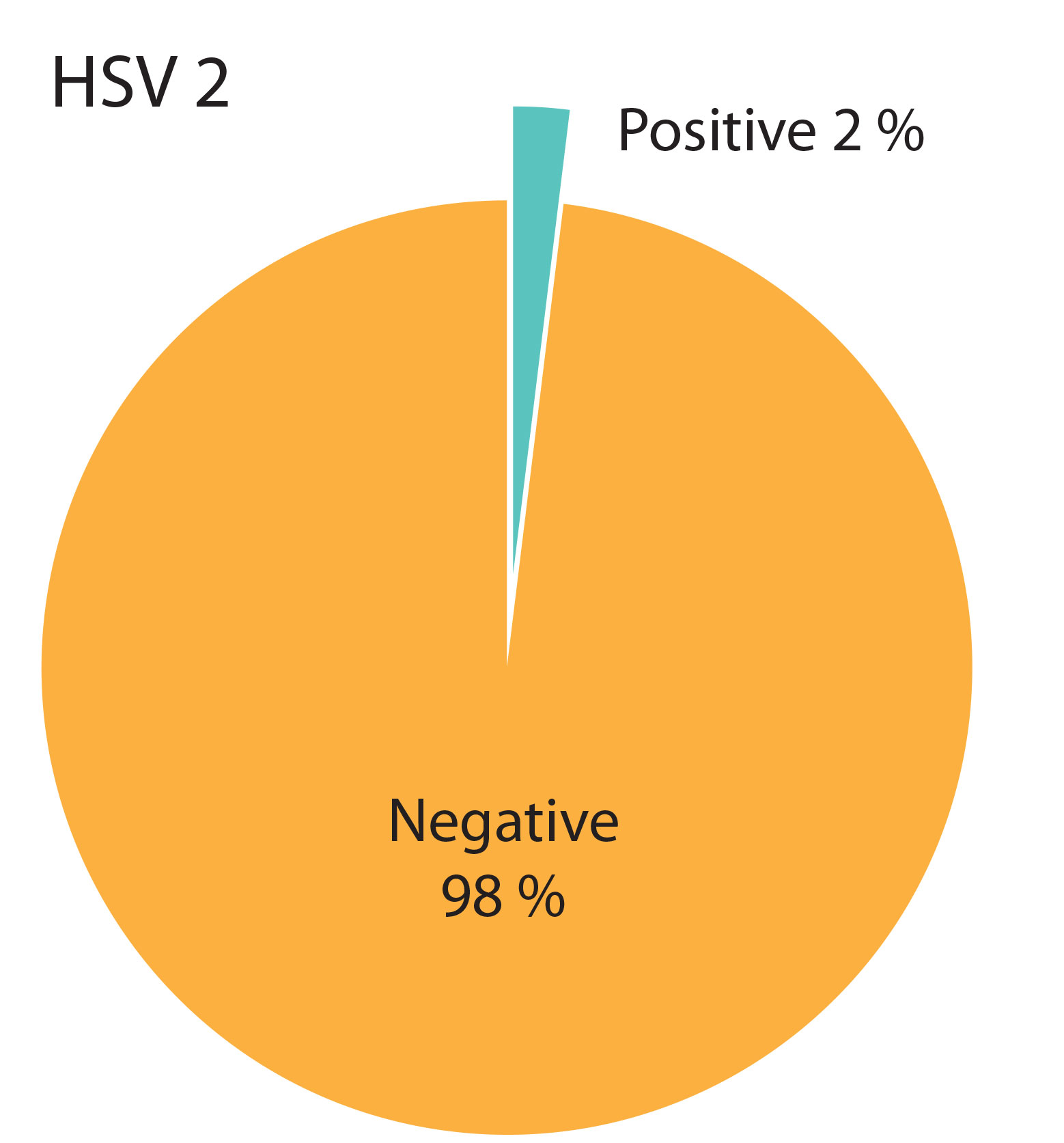
The percentage of participants with positive and negative anti-HSV antibody results is shown in the diagrams (Figure 2, Figure 3). Positive anti-HSV-1 antibody results were found in 25% of male and 30% of female students (Table 1). There was no statistically significant difference in the prevalence of positive results between genders (p=0.620). Positive anti-HSV-2 IgG antibody results were not present in any male student and in only 2% of female students. No statistically significant difference in the prevalence of positive results between genders was found (p=1.000).
Figure 2. Percentage of participants with positive and negative findings of anti-HSV1 antibodies (29% positive and 71% negative for anti-HSV-1 antibodies of IgG class).
Figure 3. Percentage of participants with positive and negative findings of anti-HSV2 antibodies (2% positive and 98% negative for anti-HSV-2 antibodies of IgG class).
Table 1. Tabular representation of positive results for anti-HSV antibodies by gender of participants.
The average titer of anti-HSV-1 IgG antibodies in positive male students was 114.6±44.9, and in female students, it was 92.8±36.6. There was no statistically significant difference in antibody titer between male and female students (p=0.225).
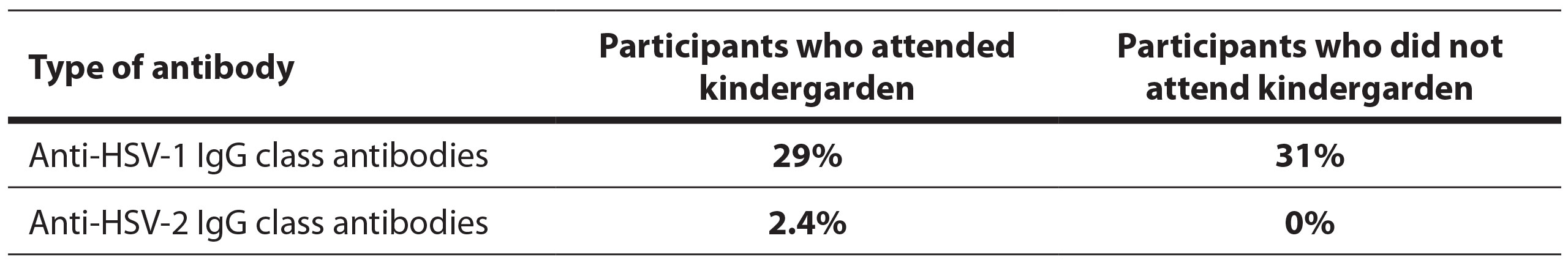
3.2. Results by attendance of kindergarden and school differences
Positive anti-HSV-1 IgG antibody results were present in 29% of students who attended and 31% of those who did not attend kindergarden (Table 2). There was no statistically significant difference in the prevalence of positive results between students who attended and those who did not attend kindergarden (p=0.829). Positive anti-HSV-2 antibody results were present in 2.4% of students who attended kindergarden and in none of those who did not attend. There was no statistically significant difference in the prevalence of positive results between these groups (p=1.000).
Table 2. Tabular representation of positive results for anti-HSV antibodies based on whether participants attended preschool institutions or not.
The average antibody titer in positive students who attended preschool was 98.4±40.8, and in those who did not attend preschool, it was 91.81±29.4. There was no statistically significant difference in antibody titer between these groups (p=0.736).
4. Discussion
This is the first study in our country to examine the seroprevalence of anti-HSV IgG class antibodies among medical students (Zhu and Viejo-Borbolla, 2021). Anti-HSV-1 IgG class antibodies were present in 29% of participants, and anti-HSV-2 IgG class antibodies were present in 2% of participants.
The results of our study show a significantly lower seroprevalence of anti-HSV-1 IgG class antibodies compared to published results from other countries. In Australia, approximately 80% of the population is infected with herpes simplex virus type 1 (HSV-1). In other countries, such as Western European countries, the United States, or Canada, the percentages are lower (74%, 58%, 51%) (AlMukdad et al. 2023).
According to our results, seroprevalence did not differ significantly between genders, which aligns with the global pattern. There are also no reports in the literature indicating greater sensitivity or exposure of one gender over the other concerning HSV-1 (AlMukdad et al. 2023).
Published data indicate a lower seroprevalence of anti-HSV-1 IgG class antibodies in the adolescent population compared to the adult population. In Australia and Europe, the seroprevalence among those under 35 years of age is 67%, while the seroprevalence among those over 35 years of age is 81.5% (AlMukdad et al. 2023). Among our respondents, the seroprevalence is much lower. These data suggest that primary contact with HSV-1 likely occurs later in life. The explanation for later primary contact with the virus includes changes in social behavior during adolescence and early adulthood, such as closer social contacts, sharing glasses or eating utensils, and direct contact through kissing (Korr et al. 2017).
Most medical students attended kindergarten (84%), and the positive rate for anti-HSV-1 antibodies was very similar between those who attended and those who did not attend kindergarden (29%-31%). This suggests that primary infection occurs independently of collective living, and attending kindergarden does not increase the risk of primary infection.
In the last three decades, the estimated seroprevalence of anti-HSV-2 antibodies in the adult population in Europe averages about 12%. This value is similar to that in Asia but lower compared to Africa, Latin America, the United States, and the Middle East and North Africa region (Samies et al. 2020; AlMukdad et al. 2023). Women are biologically more susceptible to HSV-2 infection during sexual intercourse due to the larger mucosal surface area in the genital region, which increases contact with an infected partner. Pregnancy can also increase susceptibility to HSV-2 infection, especially if a woman has her first contact with the virus during pregnancy. It is important to note that these factors are interactive, and the individual risk of infection depends on multiple factors, including biological, psychological, and sociocultural aspects. Prevention and education about safe sexual practices play a crucial role in reducing the risk of HSV-2 infections in both genders (Korr et al. 2017; Looker 2005).
In our study, only 2% of participants were seropositive, but the study population was younger compared to the subjects in the previously mentioned literature data. The seroprevalence of anti-HSV-2 IgG class antibodies in most European countries is higher in women than in men, so the results of our study with both positive samples in the female population correlate with the literature data (Alareeki et al. 2022). The seroprevalence rate among women is significant due to the possible transmission to the fetus during pregnancy, leading to congenital and perinatal infections.
Perinatal infections with HSV-1 and HSV-2 are a cause of potentially complicated infections or life-threatening conditions for newborns. Neonatal herpes and encephalitis are among the rare conditions that can result in severe consequences and death of the newborn, characterized by high morbidity and mortality (Alareeki et al. 2022; Cupic 2022). Because HSV-2 infections are asymptomatic or mild, it is very important to perform certain diagnostic tests for pregnant women, such as the TORCH test. The TORCH test is a very useful test that allows to determine whether a pregnant woman has previously been exposed to pathogens that can be life-threatening for the newborn and whether she has protective antibodies against them (Connolly et al. 2020; Looker 2005; Fuchs et al. 2021; Johnston et al. 2011; Deftereou et al. 2022).
In immunocompromised states (post-transplantation, long-term use of corticosteroids), HSV-1 and HSV-2 infections can lead to disseminated infection. This might result in severe forms of disease in some organs, including the brain (herpetic encephalitis), eyes (herpetic keratitis), and liver (herpetic hepatitis). Immunosuppressed patients may have atypical symptoms of HSV-1 and HSV-2 infection, which could complicate diagnosis and treatment (Zhu and Viejo-Borbolla, 2021; Valerio and Lin, 2019).
5. Conclusion
The results of this study indicate a significantly lower seroprevalence of anti-HSV-1 and anti-HSV-2 IgG class antibodies among medical students in Belgrade compared to global data. Given the lack of a prophylactic vaccine, the seronegative population may develop complications of primary infection later in life, especially those who might have an immunosuppressed condition. The seronegative female population is at an exceptionally high risk of fetal or perinatal viral transmission during pregnancy.
Acknowledgement: This manuscript was performed as part of a project „Cross sectional study of seroprevalence of teratogenic and MMR vaccine covered viral infections including VZV, measles morbillivirus, mumps virus, rubella virus, CMV, HSV1 and HSV2 in young adults in Serbia“, number 2641/1, at Faculty of Medicine, University of Belgrade.
References:
Ahmad, I., & Wilson, D. W. (2020). HSV-1 cytoplasmic envelopment and egress. International Journal of Molecular Sciences, 21(17), 5969.
Alareeki, A., Osman, A. M., Khandakji, M. N., Looker, K. J., Harfouche, M., & Abu-Raddad, L. J. (2022). Epidemiology of herpes simplex virus type 2 in Europe: systematic review, meta-analyses, and meta-regressions. The Lancet Regional Health – Europe, 25, 100558.
AlMukdad, S., Harfouche, M., Farooqui, U. S., Aldos, L., & Abu-Raddad, L. J. (2023). Epidemiology of herpes simplex virus type 1 and genital herpes in Australia and New Zealand: systematic review, meta-analyses and meta-regressions. Epidemiology and Infection, 151.
Connolly, S. A., Jardetzky, T. S., & Longnecker, R. (2020). The structural basis of herpesvirus entry. Nature Reviews Microbiology, 19(2), 110–121.
Cupic, M. (2022). Herpesviridae. In B.S., S.M., T.J. (Eds). Medicinska mikrobiologija (3rd ed., Vol. 1, pp. 497-508). Libri medicorum.
Deftereou, T., Trypidi, A., Alexiadi, C. A., Theotokis, P., Manthou, M. E., Meditskou, S., Simopoulou, M., & Lambropoulou, M. (2022). Congenital herpes simplex virus: A histopathological view of the placenta. Cureus.
Fuchs, F. E., Pauly, M., Black, A. P., & Hübschen, J. M. (2021). Seroprevalence of TORCH pathogens in Southeast Asia. Microorganisms, 9(3), 574.
Johnston, C., Koelle, D. M., & Wald, A. (2011). HSV-2: in pursuit of a vaccine. Journal of Clinical Investigation, 121(12), 4600–4609.
Korr, G., Thamm, M., Czogiel, I., Poethko-Mueller, C., Bremer, V., & Jansen, K. (2017). Decreasing seroprevalence of herpes simplex virus type 1 and type 2 in Germany leaves many people susceptible to genital infection: time to raise awareness and enhance control. BMC Infectious Diseases, 17(1).
Looker, K. J. (2005). A systematic review of the epidemiology and interaction of herpes simplex virus types 1 and 2. Sexually Transmitted Infections, 81(2), 103–107.
Sadowski, L. A., Upadhyay, R., Greeley, Z. W., & Margulies, B. J. (2021). Current Drugs to Treat Infections with Herpes Simplex Viruses-1 and -2. Viruses, 13(7), 1228.
Samies, N. L., & James, S. H. (2020). Prevention and treatment of neonatal herpes simplex virus infection. Antiviral Research, 176, 104721.
Singh, N., & Tscharke, D. C. (2019). Herpes simplex virus latency is noisier the closer we look. Journal of Virology, 94(4).
Valerio, G. S., & Lin, C. C. (2019). Ocular manifestations of herpes simplex virus. Current Opinion in Ophthalmology, 30(6), 525–531.
Whitley, R., & Baines, J. (2018). Clinical management of herpes simplex virus infections: past, present, and future. F1000Research, 7, 1726.
Zhu, S., & Viejo-Borbolla, A. (2021). Pathogenesis and virulence of herpes simplex virus. Virulence, 12(1), 2670–2702.