1. Introduction
Streptococcus pneumoniae (pneumococcus) is an opportunistic Gram-positive coccus that colonizes the upper respiratory tract of 11-93% of humans (Yildirim et al. 2015). Pneumococcal infections can range from noninvasive (acute otitis media, sinusitis, and non-bacteremic pneumonia) to invasive (bacteremic pneumonia, meningitis, and sepsis). While invasive infections are associated with worse outcomes, they are less common than non-invasive infections, which are highly prevalent, often require antibiotics, and impose a significant medical burden. Pneumococcus is the most commonly isolated pathogen in patients with community-acquired pneumonia with a mortality rate ranging from 6.4% to more than 40% depending on the medical setting (Blasi et al. 2012). It is estimated that only 10-30% of pneumonia caused by pneumococci are accompanied by bacteriemia thus being classified as invasive (Blasi et al. 2012). Acute otitis media (AOM) is the leading cause of antibiotic prescriptions for children, with over 10 million prescriptions in the United States annually. S. pneumoniae, along with Hemophilus influenzae and Moraxella catarrhalis, is one of the leading bacterial causes of AOM in children worldwide. It is estimated that 60% of children experience AOM before their third birthday (El Feghaly et al. 2023).
Pneumococcus is divided into over 100 serotypes, based on the structure of its polysaccharide capsule, which is its main virulence factor (Ganaie et al. 2023). The immunogenic capsular antigen is the target of the hosts immune system, especially humoral. Introducing the pneumococcal conjugate vaccines (PCVs), which contain a polysaccharide antigen conjugated to protein, has reduced the incidence of infections caused by vaccine serotypes (Yildirim et al. 2015).
However, the introduction of the PCV7 induced a change in the circulating population of pneumococcal serotypes. While vaccinal serotypes declined, non-vaccinal serotypes unaffected by vaccinal humoral immunity increased, leading to the overall rise in the incidence of pneumococcal infections (Yildirim et al. 2015, Richter et al. 2013, Horácio et al. 2014). This serotype replacement highlighted the need to introduce higher valent PCVs (PCV10, PCV13, PCV15), while simultaneously monitoring the current pool of circulating serotypes to estimate their vaccinal coverage. In the Republic of Serbia, PCV10 was introduced into the immunization schedule in 2018, replaced by PCV13 in 2022, and reinstated in 2024. According to the National Institute of Public Health “Milan Jovanovic Batut” in 2022 coverage of PCV13 in the first year of life was 90.5% (Извештај о спроведеној имунизацији на територији Републике Срије у 2022. години, 2023).
Pneumococcus is no exception in the prevalence of antimicrobial resistance, where macrolide -resistant Streptococcus pneumoniae (MRSP) is recognized by the WHO as a medium-priority pathogen to guide and promote research and development of new antibiotics (WHO, 2024). In Serbia, high levels of pneumococcal non-susceptibility (NS) have been detected against commonly used antibiotics for treating pneumococcal infections, including beta-lactams (such as penicillin, and third-generation cephalosporins) and macrolides, with 47.6%, 16.5% and 40.4% respectively (Opavski et al. 2023).
In Serbia, there is monitoring of antibiotic sensitivity and serotypes of invasive isolates of S. pneumoniae, but there is a lack of sufficient data on non-invasive isolates. This study aimed to test the distribution of serotypes and antibiotic susceptibility of S. pneumoniae isolates causing non-invasive infections collected in the post-vaccine period, in 2022 in Belgrade.
- Materials and Methods
The study included S. pneumoniae isolates causing non-invasive infections from the collection of the NRL for streptococci, Institute of Microbiology and Immunology, Faculty of Medicine in Belgrade. Isolates were collected from January 1st to December 31st, 2022. The strains were identified in the MediGroup Health System microbiology laboratory in Belgrade and then sent to the NRL for streptococci. Pure cultures of pneumococcal strains were stored until further research in skim milk broth (HiMedia, India) at -70°C.
2.1. Molecular identification of Streptococcus pneumoniae
Isolations of DNA was performed using TRIS-SDS-EDTA lysis buffer. After lysis, suspensions were incubated for 20 minutes at room temperature and 5 minutes at 90°C. After isolating bacterial DNA using cold absolute ethanol and centrifugation, DNA was resuspended in 120 µL of elution buffer.
Confirmation of the identification of S. pneumoniae strains was performed by detection of the cpsA gene, using the PCR (Jourdain et al. 2011). The reaction mixture with a volume of 25 µL contained: 12.5 µl of Master mix (Genaxxon bioscience, Germany), 0.5 µl of each primer with a concentration of 20 µmol/l (Thermo Fisher Scientific, USA), 6.5 µl of water and 5 µl of bacterial DNA. PCR products were visualized in 2% agarose gel.
2.2. Capsular typing of Streptococcus pneumoniae isolates
Typing of clinical isolates of S. pneumoniae was performed by the multiplex PCR method following the protocol of Jourdain et al (2011). A total of 7 reaction mixtures were prepared for each isolate. Reaction mixtures with a volume of 25 µL contained 12.5 µl of Master Mix (Genaxxon bioscience, Germany), primers of different serotypes at a concentration of 20 µmol/l (Thermo Fisher Scientific, USA), 5 µl of bacterial DNA, and water to the final volume. The conditions under which amplification took place were: initial denaturation at 94°C for 30 seconds; 30 cycles, denaturation at 94°C for 45 seconds, annealing at 54°C for 45 seconds and elongation at 65°C for 150 seconds; and final elongation at 72°C for 10 minutes. After the PCR, the products were applied to horizontal gel electrophoresis in 2% agarose gel.
For isolates that couldn’t be typed using PCR, Quellung reaction, using the chessboard system and specific sera (Statens Serum Institute, Denmark) was done.
2.3. Antibiotic susceptibility testing
The susceptibility of S. pneumoniae isolates was tested by the disc diffusion method according to the standards of the European Committee on Antimicrobial Susceptibility Testing (EUCAST, 2024). Susceptibility was tested to oxacillin (1μg), erythromycin (15μg), chloramphenicol (30μg), clindamycin (2μg), norfloxacin (10μg), tetracycline (30μg), trimethoprim/sulfamethoxazole (1.25-23.75μg) and vancomycin (5μg) (bioRad, USA). In isolates resistant to oxacillin, the minimum inhibitory concentrations (MIC) for penicillin and ceftriaxone were determined using the gradient test (Liofilchem, Italy) according to the EUCAST standard (EUCAST, 2024). Isolates were classified as penicillin or ceftriaxone non-susceptible S. pneumoniae (PNSP and CNSP) when MICs were higher than 0.06 mg/L for penicillin and higher than 0.5 mg/L for ceftriaxone. The phenotype of macrolide resistance was further tested using the double disc diffusion method with discs of erythromycin (15μg) and clindamycin (2μg). Isolates were classified as multidrug-resistant (MDR) when non-susceptibility or resistance to three or more antibiotic classes was exhibited (Cillóniz et al. 2018).
2.4 Statistical analysis
Statistical analyzes were performed in EZR software (R Commander Version 2.7-1). The chi-square test or Fisher’s exact probability test, was used to assess the statistical significance of the difference between the nominal categories. The difference was considered statistically significant for p<0.05.
2. Results
During the one-year period, a total of 155 S. pneumoniae isolates causing non-invasive infections were collected. Most were isolated from middle ear aspirates (n=110; 70.97%), while the rest were from tracheal aspirates (n=18; 11.6%) and sputum (n=17; 11%); sinus aspirates (n=4; 2.6%) and eye swabs (n=6; 3.9%). Isolates were collected from 83 (53.6%) male patients with a median age of 4 years (1 month to 50 years) and 72 (46.4%) female patients with a median age of 4 years (2 months to 70 years). According to the number of years, the patients were classified in the following ranks: ≤1 (n=26; 16.7%), >1-≤5 (n=76; 49%), >5-≤18 (35; 22.6%), >18 (n=18; 11.6%).
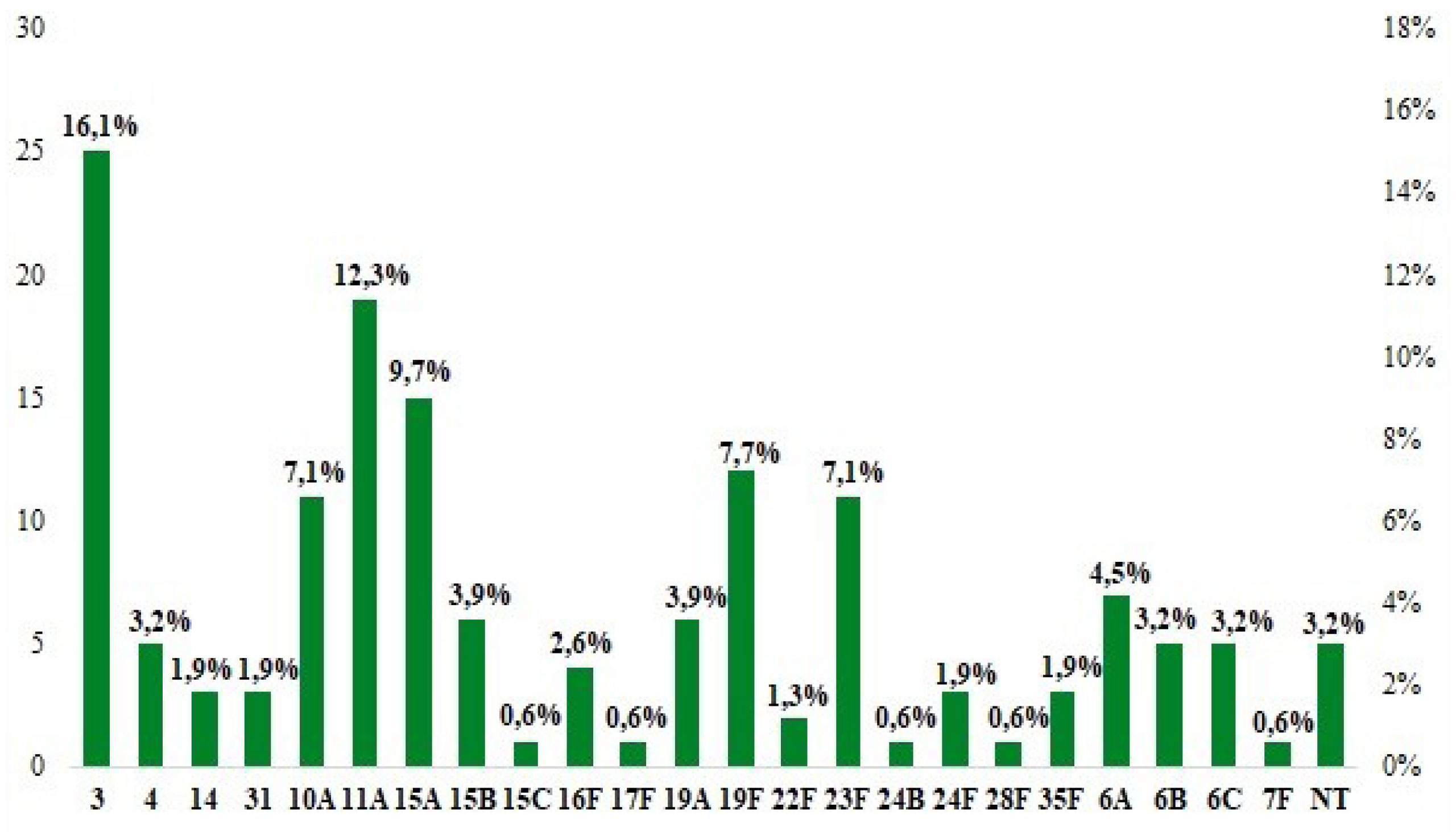
Twenty-three different serotypes were detected (Figure 1), of which the most frequent were: 3 (n=25; 16.13%), 11A (n=19; 12.26%), 15A (n=15; 9.68%), 19F ( n=12; 7.7%) and 10A (n=11; 7.1%) which included more than half of all isolates. In the age ranges >1-≤5 and >5-≤18 the most frequent serotypes were 11A (n=13; 17.1%) and 3 (n=13; 37.1%) respectively, while in the age range ≤1 the most frequent were 15A and 23F (n=4 each; 15.4%). The frequency of non-typable serotypes was 3.2% (n=5).
Figure 1. Serotype distribution of non-invasive Streptococcus pneumoniae isolates collected in 2022.
Resistance to erythromycin was the most prevalent (n=43; 27.7%), while reduced susceptibility to penicillin (both resistant and intermediate) was found in 18.1% of isolates, of which 2 (1.3%) were resistant, and 26 (16.8%) were susceptible with increased exposure. Seven (4.52%) isolates showed susceptibility to ceftriaxone with increased exposure. Additionally, 26 (16.8%) isolates were resistant to trimethoprim/sulfamethoxazole, and 42 (27.1%) isolates were resistant to tetracycline. Only one isolate (0.7%) showed resistance to norfloxacin, while no resistance to vancomycin was detected in the tested isolates.
The most common phenotype of macrolide resistance was cMLS found in 39 (90.7%) of the erythromycin-resistant isolates, while iMLS and M phenotype were equally represented with two isolates each (4.7%).
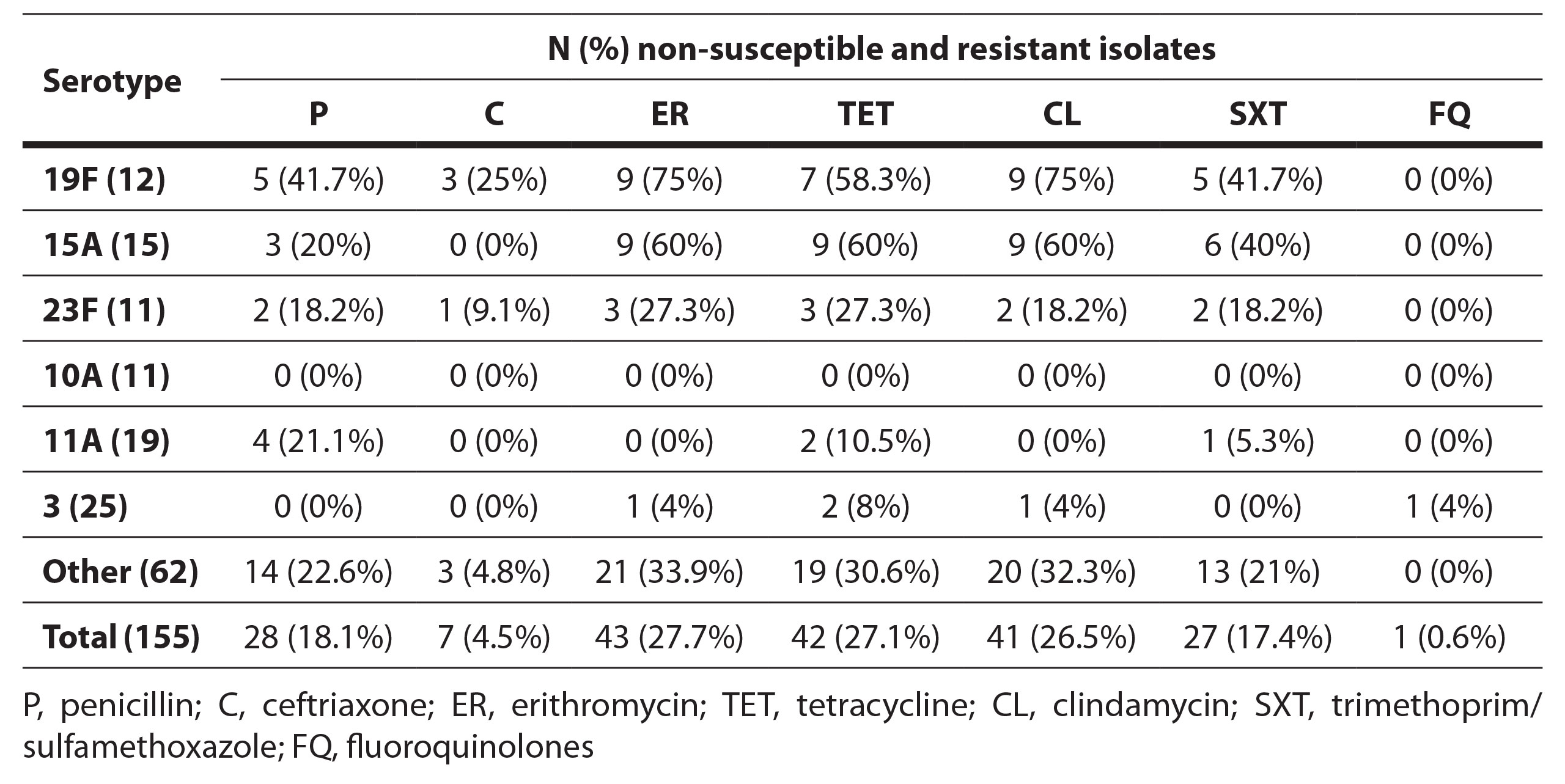
The frequency of resistance of the most common serotypes is shown in Table 1. 19F showed the highest percentages of resistance and was statistically significantly more non-susceptible to penicillin (41.6%), ceftriaxone (25%), erythromycin (75%), tetracycline (53.8%) and clindamycin (75%) compared to other serotypes (p<0.05). High frequencies of simultaneous resistance were detected in serotype 15A, which was statistically more resistant to erythromycin (60%), tetracycline (60%), and clindamycin (60%) compared to other serotypes (p<0.05) and 23F (27.3% resistant to erythromycin and tetracycline and 18.2% non-susceptible to penicillin, clindamycin and trimethoprim-sulfamethoxazole). Serotypes 10A and 3 were statistically more susceptible to penicillin, erythromycin, clindamycin, and tetracycline, while serotype 11A was more susceptible to erythromycin, clindamycin, and tetracycline (p<0.05).
Table 1. Frequency of antibiotic resistance in sixt most frequent non-invasive Streptococcus pneumoniae serotypes in this study (19F, 15A, 23F, 10A, 11A, 3).
The frequency of multidrug resistance (MDR) isolates was 25.2% (n=39). The most frequent MDR serotypes were 15A (n=9; 23.1%), 19F (n=7; 17.9%), 19A and 6B (n=4 each; 10.3%) and 23F, 24F, and 6A (n=3; 7.7%), while two (5.1%) isolates were non-typable.
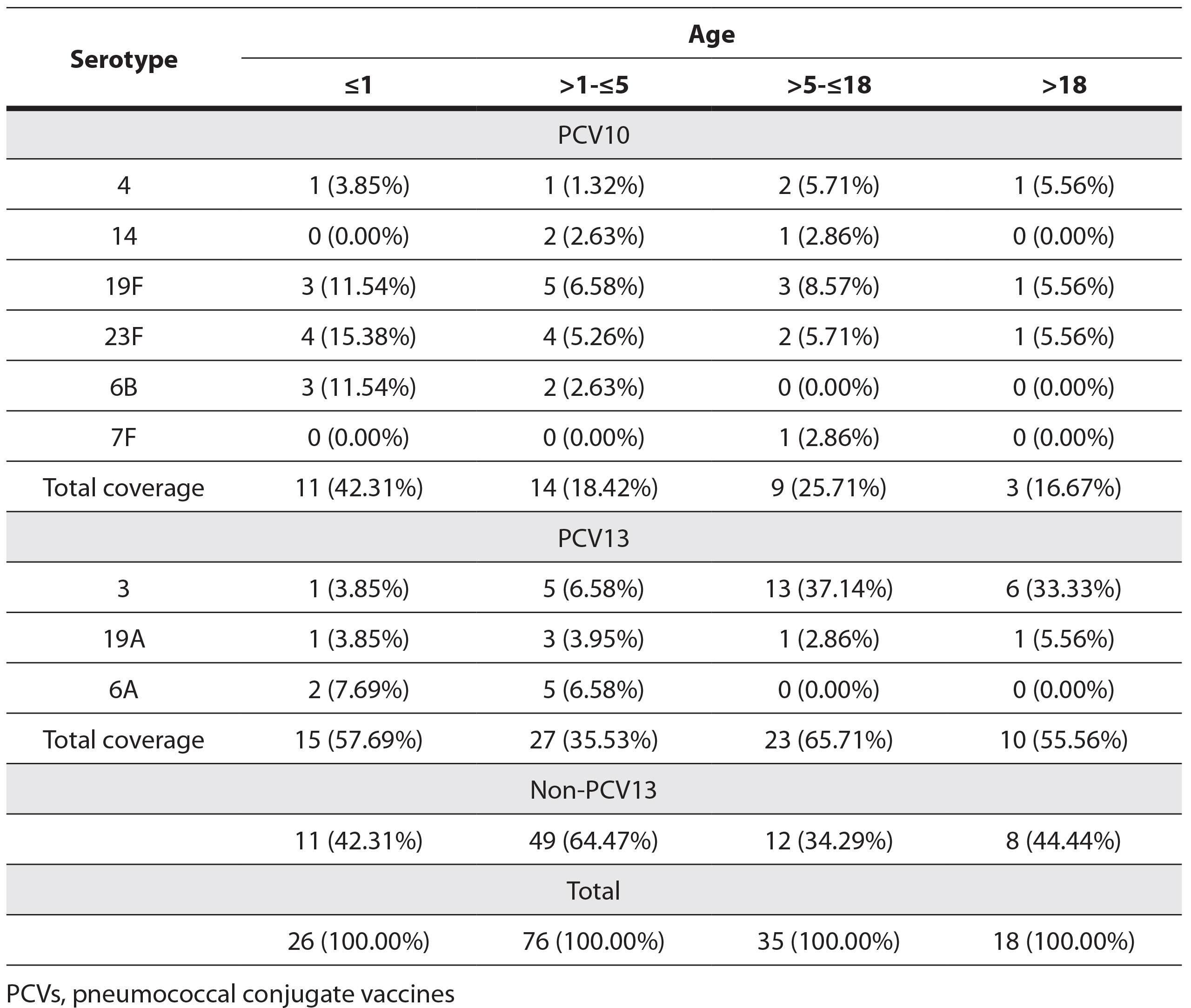
The total serotype coverage of PCV10 and PCV13 was 23.89% and 48.41%, respectively. The distribution of the most common serotypes and PCV10 and PCV13 serotype coverage by age is shown in Table 2.
Table 2. Distribution of the most common serotypes among age groups and PCV10 and PCV13 serotypes of non-invasive Streptococcus pneumoniae
4. Discussion
Among S. pneumoniae isolates causing non-invasive infections in the Serbian capital in 2022, four years after the introduction of PCV into the immunization schedule, the most frequent serotypes were 3, 11A, 15A, 19F, 10A, and 23F. In children ≤5 years of age, a similar distribution of serotypes was observed, except for serotype 3, which was slightly less prevalent (5.9% of isolates compared to 16.1% in the total sample). In contrast, among patients >5 years old, serotype 3 was the most frequent and represented 35.9% of the isolates in this group. These results indicate that circulating serotypes, such as 3 and 19F correspond to the pre-vaccine distribution of serotypes in our country (3 and 19F) (Petrović et al 2016; Opavski et al. 2023), as well as in neighboring like Bulgaria, Romania (19F and 23F) and also the Russian Federation (19F, 23F and 3) (Falup-Pecurariu et al. 2013; Setchanova et al. 2013; Mayanskiy et al. 2014) While serotypes 14, 9V, and 9N were not frequently detected in this study, they were prominently reported in studies conducted prior the introduction of vaccines (Falup-Pecurariu et al. 2013; Setchanova et al. 2013; Mayanskiy et al. 2014; Petrović et al 2016; Opavski et al. 2023). Following the introduction of the PCV vaccine, non-vaccine serotypes identified in this study (10A, 11A, and 15A) were significantly more prevalent compared to their occurrence in countries such as Portugal and South Korea. (Horácio et al. 2014; Park et al. 2019). This pattern of serotype distribution may be attributed to the selective pressure from the recently introduced conjugate vaccines, resulting in the emergence of non-vaccine serotypes. However, this process remains incomplete due to the short period since the vaccine introduction and reduced vaccine coverage during the COVID-19 pandemic. Serotype 3, the most frequent identified serotype in this study, was also highly prevalent among invasive isolates in Serbia (Opavski et al. 2023). This serotype was widely reported in certain countries, such as Portugal and the USA, even before the introduction of PCV vaccines (Richter et al. 2013; Horácio et al. 2014). Moreover, this serotype is being increasingly recognized as the major cause of both non-invasive and invasive pneumonia in countries that have been using PCV13 (which contains this serotype) for many years (Silva-Costa et al. 2023). Unlike other serotypes included in the vaccine, serotype 3 behaves as an exception, as its frequency of isolation does not decline significantly in the post-vaccination period (Horácio et al. 2014; Yildirim et al. 2015; Silva-Costa et al. 2023). The persistence of serotype 3 is explained by its specific capsular polysaccharide, and the release of capsule fragments after the binding of type-specific antibodies, allowing it to avoid the immune response. The antibody levels specific for serotype 3, which arise after administration of PCV13, are insufficient to provide effective protection against this serotype (Choi et al. 2015; Luck et al. 2020).
Compared to the resistance rate of pneumococci to erythromycin in the pre-vaccination period in Serbia (65.5% of non-invasive and 40.4% of invasive isolates), the resistance rate of the tested samples is significantly lower (27.7%) (Jovanović et al. 2017; Opavski et al. 2023). In comparison to other countries, during the post-vaccination period (41.1% in the USA, 84% in South Korea), the frequency of erythromycin resistance to is also significantly lower (Park et al. 2019; Mohanty et al. 2023). Similarly, a significantly higher percentage of reduced susceptibility to penicillin was found in the pre-vaccination period in Serbia, with rates of 65.6% among non-invasive and 47.5% among invasive pneumococcal isolates, compared to the current study where reduced susceptibility was 18.1% (Jovanović et al. 2017; Opavski et al. 2023). A similar frequency was detected in Romania in 2022, with approximately 20% of isolates showing reduced susceptibility (Gavrilovici et al. 2022), while the rate remains lower compared to other studies (42.4% in the USA and 38.7% in South Korea) (Park et al. 2019; Mohanty et al. 2023). Despite the decline in resistance rates following vaccine introduction in Serbia and other countries, such as Finland, resistance levels in this study population remain high and underscore the need for continued monitoring (Sihvonen et al. 2017).
The frequency of multidrug-resistant strains is 25.2%, which is the most consistent with the results from Russia during pre-vaccine period (22% of isolates) (Mayanskiy et al. 2014). However, it is significantly lower compared to other countries in the region, such as Romania (68.7% pre-vaccination and 82.85% post-vaccination) and Bulgaria (54% post-vaccination) (Falup-Pecurariu et al. 2013; Setchanova et al. 2018; Gavrilovici et al. 2022). Surprisingly, the frequency of invasive MDR isolates in this study was higher compared to the frequency of multidrug resistance in our country in the pre-vaccine period, which was 20% (Opavski et al. 2023). The most prevalent serotypes among MDR isolates were 15A, 19F, 19A, and 6B, which were also identified in Serbia before vaccine introduction (19F, 19A, and 6B) as well as in South Korea post-vaccination (15A, 19F, and 19A) (Park et al. 2019; Opavski et al. 2023). In contrast to this study, where serotype 11A was one of the most susceptible isolates, recent studies from Portugal (Silva-Costa et al. 2023) reported this serotype with a high prevalence of resistance. Serotype 14 was the third most frequent among MDR serotypes in our country and Russia (Mayanskiy et al. 2014; Opavski et al. 2023) before vaccine introduction accounted for less than 2% of the isolates in this study.
Coverage of serotypes PCV10 and PCV13 vaccines in children ≤5 years of this study (24.5% and 41.2%) was significantly lower compared to the coverage of invasive isolates in the pre-vaccine period in our country (76% and 88%) (Petrović et al. 2016). A decrease in the detection of vaccine serotypes after the introduction of the vaccine was also detected in other countries (in the USA from 43.4% to 27.1%) (Richter et al. 2013).
The limitations of this study are the small number of available isolates of non-invasive pneumococcus during one year, considering that isolates of non-invasive S. pneumoniae are not collected systemically in our country and represent the available part of the National Reference Laboratory (NRL) collection for streptococci.
5. Conclusions
The most prevalent serotypes of S. pneumoniae isolated from non-invasive infections in this study were 3 and 11A, with a notable dominance of non-PCV13 serotypes in the tested population. As anticipated, the prevalence of PCV10 and PCV13 serotypes was low (23.89% and 48.41%, respectively), given that the study was conducted in the post-PCV period, observing the effect of PCV on noninvasive isolates. The resistance rates among our isolates were notably high, with 25% exhibiting an MDR phenotype. Therefore, changes in serotype distribution and the prevalence of antimicrobial resistance in non-invasive pneumococcal strains should be closely monitored following the implementation of PCV and subsequent changes to the vaccine.
Funding: Ministry of Science, Technological Development, Innovation of the Republic of Serbia, grant No. 51-03-66/2024-03/200110, subgrant “Multidrug-Resistant Bacteria in the Hospital Environment in Serbia: Prevalence, Circulating Clones, Genetic Basis of Antibiotic Resistance, and Virulence Factors”.
Informed Consent Statement: Not applicable.
Data Availability Statement: All data are presented in the article.
Conflicts of Interest: The authors declare no conflicts of interest.
References:
Blasi, F., Mantero, M., Santus, P., & Tarsia, P. (2012). Understanding the burden of pneumococcal disease in adults. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases, 18 Suppl 5, 7–14.
Choi, E. H., Zhang, F., Lu, Y. J., & Malley, R. (2015). Capsular Polysaccharide (CPS) Release by Serotype 3 Pneumococcal Strains Reduces the Protective Effect of Anti-Type 3 CPS Antibodies. Clinical and vaccine immunology: CVI, 23(2), 162–167.
Cillóniz, C., Garcia-Vidal, C., Ceccato, A., & Torres, A. (2018). Antimicrobial Resistance Among Streptococcus pneumoniae. Antimicrobial Resistance in the 21st Century, 13–38
El Feghaly, R. E., Nedved, A., Katz, S. E., & Frost, H. M. (2023). New insights into the treatment of acute otitis media. Expert review of anti-infective therapy, 21(5), 523–534.
Falup-Pecurariu, O., Leibovitz, E., Mercas, A., Bleotu, L., Zavarache, C., Porat, N., Dagan, R., & Greenberg, D. (2013). Pneumococcal acute otitis media in infants and children in central Romania, 2009-2011: microbiological characteristics and potential coverage by pneumococcal conjugate vaccines. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases, 17(9), e702–e706.
Ganaie, F. A., Saad, J. S., Lo, S. W., McGee, L., van Tonder, A. J., Hawkins, P. A., Calix, J. J., Bentley, S. D., & Nahm, M. H. (2023). Novel pneumococcal capsule type 33E results from the inactivation of glycosyltransferase WciE in vaccine type 33F. The Journal of biological chemistry, 299(9), 105085.
Gavrilovici, C., Spoială, E. L., Miron, I. C., Stârcea, I. M., Haliţchi, C. O. I., Zetu, I. N., Lupu, V. V., & Pânzaru, C. (2022). Acute Otitis Media in Children-Challenges of Antibiotic Resistance in the Post-Vaccination Era. Microorganisms, 10(8), 1598.
Horácio, A. N., Lopes, J. P., Ramirez, M., Melo-Cristino, J., & Portuguese Group for the Study of Streptococcal Infections (2014). Non-invasive pneumococcal pneumonia in Portugal–serotype distribution and antimicrobial resistance. PloS one, 9(7), e103092.
Jovanović L., Isailović K., Opavski N. (2017). Frequency of resistance to penicillin and erythromycin of pneumococcal strains that caused ottis media. Med Podml ;68(1):26-30.
Jourdain, S., Drèze, P. A., Vandeven, J., Verhaegen, J., Van Melderen, L., & Smeesters, P. R. (2011). Sequential multiplex PCR assay for determining capsular serotypes of colonizing S. pneumoniae. BMC infectious diseases, 11, 100.
Luck, J. N., Tettelin, H., & Orihuela, C. J. (2020). Sugar-Coated Killer: Serotype 3 Pneumococcal Disease. Frontiers in cellular and infection microbiology, 10, 613287.
Mayanskiy, N., Alyabieva, N., Ponomarenko, O., Lazareva, A., Katosova, L., Ivanenko, A., Kulichenko, T., Namazova-Baranova, L., & Baranov, A. (2014). Serotypes and antibiotic resistance of non-invasive Streptococcus pneumoniae circulating in pediatric hospitals in Moscow, Russia. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases, 20, 58–62.
Mohanty, S., Feemster, K., Yu, K. C., Watts, J. A., & Gupta, V. (2023). Trends in Streptococcus pneumoniae Antimicrobial Resistance in US Children: A Multicenter Evaluation. Open forum infectious diseases, 10(3), ofad098.
Opavski, N., Jovicevic, M., Kabic, J., Kekic, D., Vasiljevic, Z., Tosic, T., Medic, D., Laban, S., Ranin, L., & Gajic, I. (2023). Serotype distribution, antimicrobial susceptibility and molecular epidemiology of invasive Streptococcus pneumoniae in the nine-year period in Serbia. Frontiers in microbiology, 14, 1244366.
Park, D. C., Kim, S. H., Yong, D., Suh, I. B., Kim, Y. R., Yi, J., Song, W., Song, S. A., Moon, H. W., Lee, H. K., Park, K. U., Kim, S., Jeong, S. H., Lee, J., Jeong, J., Kim, Y. K., Lee, M., Cho, J., Kim, J. W., Shin, K. S., … Shin, J. H. (2019). Serotype Distribution and Antimicrobial Resistance of Invasive and Noninvasive Streptococcus pneumoniae Isolates in Korea between 2014 and 2016. Annals of laboratory medicine, 39(6), 537–544.
Petrović V, Šeguljev Z, Ristić M, Djekić-Malbaša J, Radosavljević B, Medić D, Mihajlović-Ukropina M, Hadnadjev M, Gajić I, Opavski N. (2016) Streptococcus pneumoniae serotype distribution in Vojvodina before the introduction of pneumococcal conjugate vaccines into the National Immunization Program. Srp Arh Celok Lek. 2016 Sep-Oct; 144(9-10):521-526.
Richter, S. S., Heilmann, K. P., Dohrn, C. L., Riahi, F., Diekema, D. J., & Doern, G. V. (2013). Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999-2011(1.). Emerging infectious diseases, 19(7), 1074–1083.
Setchanova, L., Alexandrova, A., Pencheva, D., Sirakov, I., Mihova, K., Kaneva, R., & Mitov, I. (2018). Rise of multidrug-resistant Streptococcus pneumoniae clones expressing non-vaccine serotypes among children following introduction of the 10-valent pneumococcal conjugate vaccine in Bulgaria. Journal of global antimicrobial resistance, 15, 6–11.
Setchanova, L. P., Kostyanev, T., Alexandrova, A. B., Mitov, I. G., Nashev, D., & Kantardjiev, T. (2013). Microbiological characterization of Streptococcus pneumoniae and non-typeable Haemophilus influenzae isolates as primary causes of acute otitis media in Bulgarian children before the introduction of conjugate vaccines. Annals of clinical microbiology and antimicrobials, 12, 6.
Sihvonen, R., Siira, L., Toropainen, M., Kuusela, P., & Pätäri-Sampo, A. (2017). Streptococcus pneumoniae antimicrobial resistance decreased in the Helsinki Metropolitan Area after routine 10-valent pneumococcal conjugate vaccination of infants in Finland. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology, 36(11), 2109–2116.
Silva-Costa, C., Gomes-Silva, J., Santos, A., Ramirez, M., Melo-Cristino, J., & Portuguese Group for the Study of Streptococcal Infections (2023). Adult non-invasive pneumococcal pneumonia in Portugal is dominated by serotype 3 and non-PCV13 serotypes 3-years after near universal PCV13 use in children. Frontiers in public health, 11, 1279656.
The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 14.0, 2024. http://www.eucast.org.
WHO Bacterial Priority Pathogens List, 2024: bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance. Geneva: World Health Organization; 2024. Licence: CC BY-NC-SA 3.0 IGO.
Yildirim, I., Shea, K. M., & Pelton, S. I. (2015). Pneumococcal Disease in the Era of Pneumococcal Conjugate Vaccine. Infectious disease clinics of North America, 29(4), 679–697.
Извештај о спроведеној имунизацији на територији Републике Срије у 2022. години. (2023) In Serbian. Available at: 2022izvestajOSprovedenojImunizaciji.pdf (batut.org.rs).