1. Introduction
Pertussis, also known as whooping cough, is characterized by a paroxysmal cough ending in the recognizable “whoop”. Although generally considered a mild illness, it can cause severe complications such as apnea, convulsions, and seizures. Less common manifestations are pneumonia, encephalopathy, and rarely death. Bordetella pertussis is the causative agent of pertussis, a highly contagious disease transmitted via droplet inhalation of aerosols. The disease can occur in up to 80 to 90% of non-immunized individuals in contact with an infected person. The disease is most severe in the first two years of life, with the highest mortality among children under one-year-old who are not immunized. Even newborns are not fully protected from the disease. Although as maternal antibodies pass through the placenta, protection is incomplete due to a lack of antibodies to all B. pertussis antigens (Kilgore et al. 2016). The increased incidence of pertussis is also observed in older children and incompletely vaccinated or unvaccinated adults and represents concern due to the increased risk of transmitting the infection (Melvin et al. 2024).
The World Health Organization and the Centers for Disease Control and Prevention state that there are periodic changes in the incidence of pertussis in some parts of the world. Although pertussis is a worldwide endemic, occasional outbreaks happen in different places around the world every two to five years, commonly in both developing and developed countries (Decker and Edwards, 2021; ECDC, 2022; Wirsing von König, 2017).
An estimated 136,000 cases worldwide were reported, with a modest increase in pertussis incidence (Kilgore et al. 2016). Within the epidemiological assessment of the global burden of pertussis disease, it is necessary to consider several hypothetical aspects including (i) more sensitive diagnostic tests combined with greater awareness of pertussis disease; (ii) inadequate vaccination schedules and poor adherence to vaccination recommendations; (iii) evolution of circulating pertussis strains to avoid vaccine-induced immunity (Saadatian-Elahi et al. 2016).
After reviewing the reports of infectious diseases in the Public Health Service of Belgrade, Republic of Serbia, and the laboratory-determined causative agent, it was found that from March 2023 to February 18th, 2024, there were 857 cases of whooping cough registered in Belgrade, with an incidence rate of 50.1 per 100,000 people (Institut za javno zdravlje Srbije Dr Milan Jovanovic Batut, 2023; Institut za javno zdravlje Srbije Dr Milan Jovanovic Batut, 2024; Gradski zavod za javno zdravlje Beograd, 2024).
2. Unraveling the pathogenesis: insights into mechanisms
B. pertussis is a human pathogen, and the main source of the disease is an infected individual in the catarrhal stage when many bacteria are excreted (Willey and Wood, 2019).
B. pertussis is a small, aerobic, non-motile, Gram-negative cocobacillus, classified under the Alcaligenaceae family. Although B. pertussis has been classically identified as the sole agent responsible for whooping cough, other Bordetella species (i.e., B. parapertussis and B. holmesii) have been shown to infect humans as well as animals. In humans, B. parapertussis also can cause whooping cough-like illness, although the severity of symptoms tends to be milder than those seen with B. pertussis (Kilgore et al. 2016).
The severity of the symptoms depends on several factors, including the patient’s age, strength of the immune response, and extent of systemic bacterial dissemination. In rare cases as a pertussis complication could be necrotizing bronchitis, diffuse alveolar damage, intra-alveolar hemorrhage, fibrinous edema, macrophage-rich alveolar infiltrates, lymphangiectasia, neutrophilic bronchopneumonia, and fibrin thrombi. In more severe cases, these pathological events can lead to pulmonary hypertension, respiratory failure, and even death (Kilgore et al. 2016; Melvin et al. 2014; Decker and Edwards, 2021); van der Zee and Schellekens, 2015).
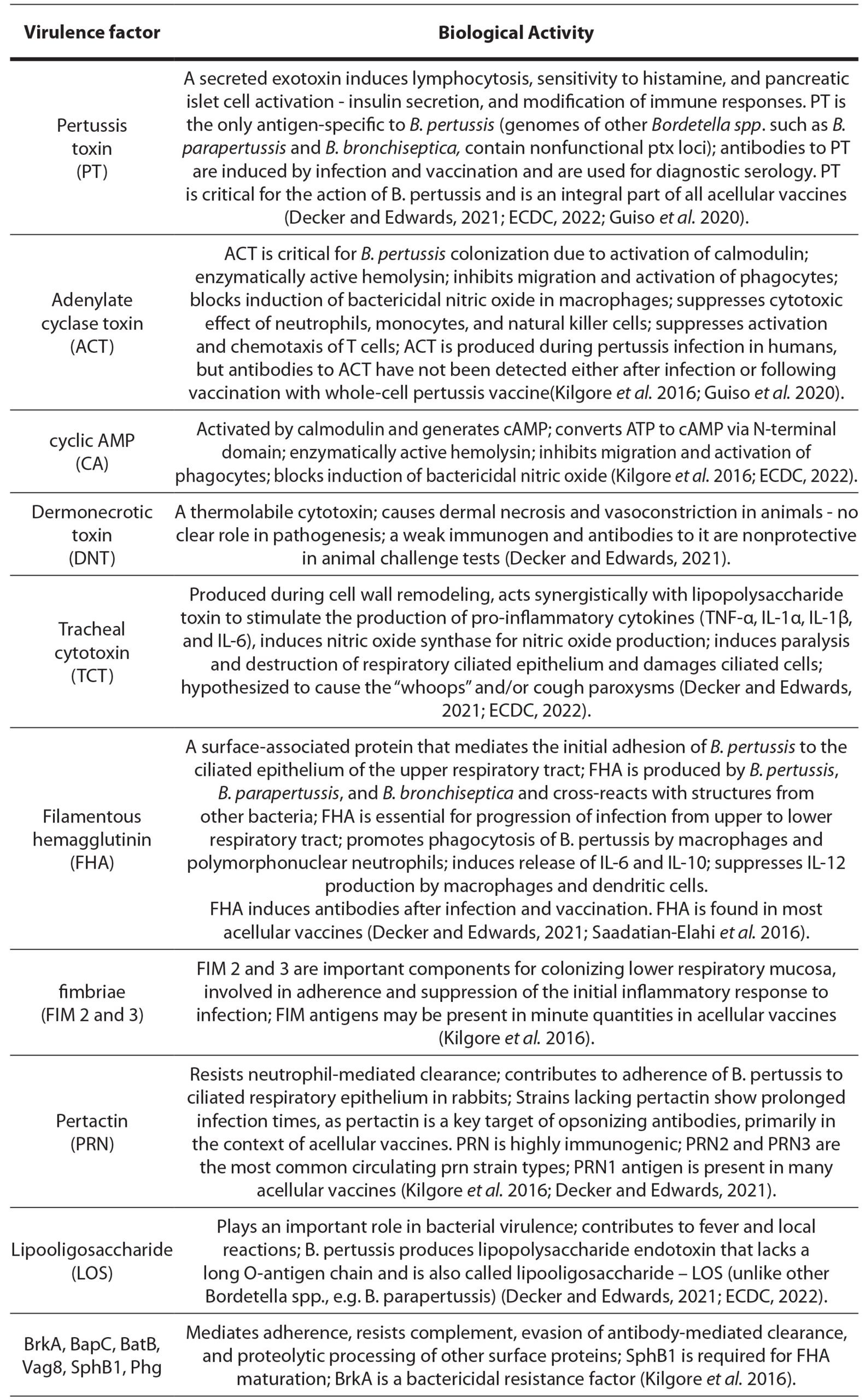
The interaction of several virulence factors of B. pertussis participates in the pathogenesis of pertussis and, in addition to the immune system, determines the clinical progression and spectrum of the disease (table 1).
Table 1. Virulence factors of Bordetella pertussis and their biological activity
The expression of several virulence factors, including PT, AC, DNT, FHA, TcfA, pertactin, FIM, BrkA, BipA, BcfA, and Vag8 is positively controlled by the BvgAS two-component signal transduction system (ECDC, 2022). This plays a crucial role in the pathogenicity of pertussis. Environmental cues like temperature changes influence the regulation of virulence factor expression once inside the human host. During transmission from person to person, B. pertussis moves from local environmental temperatures to higher body temperatures, which appear to influence the regulation of BvgAS genes (Melvin et al. 2024).
Briefly, the mechanisms of virulence of B. pertussis consist of a cascade of events initiated by the adherence of bacteria to tracheal epithelium and lungs as an essential primary step (Melvin et al. 2024). B pertussis can disrupt the immune system in a variety of ways, including by blocking complement, phagocytes, T- and B-cell responses, thanks to its proteins and toxins (Decker and Edwards, 2021). Once adherence takes place, B. pertussis cells multiply locally, resist host defense mechanisms, and cause local damage to the upper and lower respiratory tracts with systemic manifestations.
Immunological studies suggest that cellular immunity has a fundamental role to play in the immune response to pertussis. Toll-like receptor 4 (TLR4) in concert with CD4-positive type 1 T-helper cells (Th-1) and interleukin-17 (IL-17)-producing T-helper cells (Th-17) appear to play a central role in mediating protective immunity, whereas Th-1 cells alone mediate the protective immune response after recovery from B. pertussis infection. B. pertussis infection elicits a higher level of CD3 T-lymphocyte proliferation. Also, other molecules playing a role in protection against pertussis include interferon-gamma, which appears to protect against systemic dissemination, IL-12, which induces Th-1 cells, and tumor necrosis factor-alpha (TNF-A), which enhances macrophage phagocytosis of B. pertussis (Kilgore et al. 2016; Melvin et al. 2024; Decker and Edwards, 2021).
Despite recent advances in B. pertussis research in the past two decades, much remains unknown about the pathogenesis of pertussis. For instance, the precise mechanism underlying the paroxysmal cough-associated pertussis “whoops” has not been identified. Further studies on the pathogenesis of B. pertussis may also provide the basis for the design of novel antimicrobial agents that interfere with newly identified virulence mechanisms.
Immunity to pertussis, acquired through natural infection, is not permanent. Studies show that natural pertussis infection gives protection ranging from 3.5 to 30 years depending on many factors, such as the individual immunological status, and age (Kilgore et al. 2016).
3. Vaccine-Induced immunity
The re-emergence of pertussis as a global public health problem leads to the development of therapeutic forms and treatment strategies that reduce morbidity and mortality in vulnerable populations. The most important strategy is the development of vaccines with an acceptable safety profile, providing long-lasting immunity, preventing the development of severe clinical symptoms, and preventing transmission of the infection (Melvin et al. 2024).
Routine pertussis immunization has significantly reduced the incidence of the disease in the past several decades. Currently, two types of vaccines are in use for immunization against pertussis: whole-cell and acellular vaccines. They differ in their composition, with the composition varying among whole-cell vaccines depending on the strains included, while acellular vaccines may vary in the number of components included and the composition of the production strain used in their purification process. Different countries in Europe and around the world use different types of vaccines (Melvin et al. 2024; Guiso et al. 2020; Saso et al. 2021).
3.1. Whole-cell pertussis vaccine
The introduction of whole-cell pertussis (wP) vaccines in the late 1940s resulted in a reduction in morbidity and mortality caused by pertussis, and by 1970, pertussis was practically eradicated in developed countries (Guiso et al. 2020).
The manufacturing process includes cultivating bacteria in standardized liquid synthetic media, treatment of the bacteria chemically or by heat to be inactivated, and adjustment to a specific density (i.e., cell count). The manufacturing process and strain composition may vary from manufacturer to manufacturer, and the WHO has established quality and manufacturing requirements for wP vaccine batch release (Wirsing von König, 2017; WHO Annex 4, 2013).
wP vaccines contain varying amounts of whole, inactivated bacterial cells with all the antigens and virulence factors described above – such as PT, ACT, LOS, FHA, PRN, and FIM (ECDC, 2022; Edwards et al. 2023). Although the production process of wP vaccines seems straightforward and standardized, significant differences in immunogenicity and efficacy of wP vaccines from different manufacturers have been observed. For example, significant variations have been found in the amount of FHA and PT in different wP vaccines. FHA, measured as an antigen, ranges between 0 and 1.6 µg per dose, and the total biologically active PT reported ranges from 0.02 to 0.68 µg per dose. The amount of FIM2 in some wP vaccines was estimated at 4.7 µg per dose. The amount of LOS in wP vaccines ranges from 0.9 to 2.8 µg per mL. The release of LOS from cells during vaccine storage is relatively rapid; 35-50% of LOS is released in the first few weeks, and 60-80% is released after 5-6 months (Wirsing von König, 2017).
Children vaccinated with the wP vaccine may show an increase in antibody levels against FHA, PT, AGG-FIM, LPS, and outer membrane proteins (OMP), depending on the wP vaccine and immunization schedule. The degree of response was proportional to the number of doses administered. Elevated levels of antibodies to OMP and LOS have also been found in the sera of unvaccinated children, likely resulting from cross-reaction to non-pertussis antigens. Antibody responses to the given vaccine immediately after birth have also been reported. These findings are confirmed by the clinical trials conducted at two different academic centers in the USA, which showed that two commercially available wP vaccines consistently differed in their ability to induce antibody production to PT and agglutinin. wP vaccines with similar production processes differ in their antigen doses, so differences in the immunogenicity of different wP vaccines are not surprising (Decker and Edwards, 2021; Saadatian-Elahi et al. 2016).
wP are very effective in preventing disease, with reported efficacy of 85-95%. However, they were also associated with a high rate of adverse events, particularly in very young children. In the 1970s, it became recognized that a significant proportion of these events were due to a type 1 hypersensitivity reaction to the vaccine (ECDC, 2022; WHO Annex 4, 2013).
LOS is likely responsible for some of the adverse reactions in children following whole-cell pertussis immunization and has antigenic (as non-protective) and adjuvant properties. Like endotoxin in other Gram-negative organisms, LOS in wP vaccines has been significantly associated with the frequency of fever and local reactions following vaccination. LOS induces antibodies after infection and vaccination with wP vaccines but is not a declared component of acellular pertussis vaccines.
Since LOS is a potent adjuvant of the immune system, changes in the composition or concentration of LOS can affect the vaccine-induced immune response. One approach to producing less reactogenic wP vaccines is to eliminate or modify the endotoxin chemically or genetically. Attempts to eliminate or modify LOS from pertussis vaccines have already been made, but to date, a pertussis vaccine containing genetically detoxified components has not been produced (Decker and Edwards, 2021; Edwards et al. 2023). Out of all the vaccines that are regularly given, whole-cell pertussis vaccines are one of the most reactogenic. About half of DTP recipients experience minor local reactions (pain, swelling, and redness at the injection site) and systemic symptoms (fever, irritability, and sleepiness); this is five times more common than with diphtheria and tetanus (DT) vaccine (Guiso et al. 2020; Saso et al. 2021).
Concerns about reactogenicity led to a negative public perception of pertussis vaccination. Although in many countries (such as France) the effectiveness of wP vaccines has remained unchanged and at a high level for more than 30 years, the adverse effects associated with the use of wP vaccines shifted strategy towards the use of acellular vaccines (aP) in developed countries (Melvin et al. 2024; Saadatian-Elahi et al. 2016).
Reductions in hospitalizations after switching from wP to aP vaccines have been observed in Canada. In contrast, a study in rural Senegal reported that wP vaccines were more effective than two-component aP vaccines. The only European country still using whole-cell vaccine is Poland (Polak et al. 2019).
3.2. Acellular pertussis vaccine
The shifts in vaccination strategies in several countries in response to perceived high rates of adverse events from wP vaccines. Less reactogenic acellular pertussis (aP) vaccines were first developed and used in Japan about 35 years ago; then in North America, Australia, and in European countries about 15–20 years ago; and recently in other middle- and high-income countries (Kilgore et al. 2016; Saadatian-Elahi et al. 2016).
All aP vaccines are associated with significantly fewer serious adverse effects, so the replacement of wP vaccines has mainly been introduced for safety reasons. Another important advantage of aP vaccines is their reproducible manufacturing process using purified antigens and the removal of LPS and other parts of the bacterial cell wall during the purification of soluble antigenic components.
aP vaccines are licensed in most countries for primary and booster immunization. Most licensed aP vaccines contain between one and four or five separately purified antigens (PT, FHA, PRN, FIM2/3) that have been identified that play a major role in pertussis disease and protective immunity. Clinical studies in Japan, the USA, and Sweden showed that various formulations of acellular vaccines are less reactogenic than wP vaccines because the components of acellular vaccines are also at least as immunogenic as the whole-cell vaccine. Long-term monitoring of the effectiveness of aP vaccines was initiated in Sweden, starting from the completion of efficacy trials there. Although vaccines used in different parts of Sweden differed, an overall reduction in pertussis cases was maintained among all vaccinated individuals.
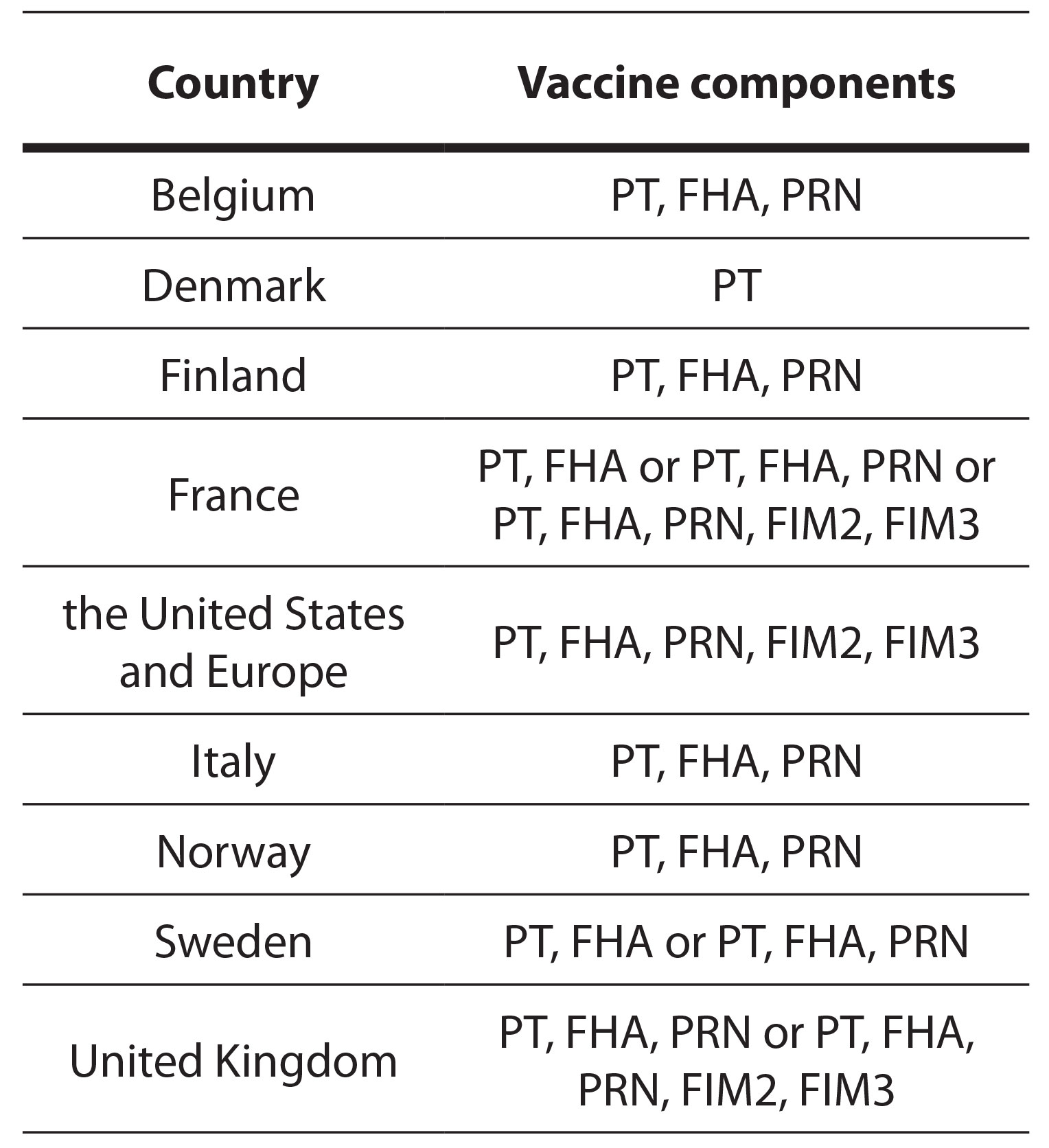
Currently, acellular vaccines dominate, although various countries use vaccines with different numbers of components. Table 2 lists manufacturers and the components of aP vaccines. For example, as of data up to 2015, Denmark used a monocomponent vaccine, while some countries, such as France, used acellular vaccines with three, four, or five components in different regions (Barkoff et al. 2018).
Table 2. List of the components of aP vaccines
Similar to infection and wP vaccination, aP vaccines not only induce antibodies against vaccine antigens but also elicit B and T cell responses. However, the cell response elicited by the aP vaccine is, to a greater or lesser extent, skewed toward a Th2 immune response, while infection and wP vaccination result in an immune response directed toward Th1. Animal models have shown that memory CD4+ T helper (Th)1 and Th17 cell phenotypes facilitate long-term protection induced by natural infection as well as wP immunization. In contrast, aP vaccination is associated with a dominant Th2 response in humans. There is evidence to suggest that acellular pertussis vaccines primarily trigger a type of immune response known as the Th-2 CD4-positive cellular immune response. However, other studies have shown that a Th-1 or a mixed Th-1/Th-2 response can also occur after a three-component acellular pertussis vaccination. It has been found that the Th-2 immune response is not as effective as the Th-1/Th-17 response, which could explain why acellular vaccines offer less long-term protection in the absence of a Th-1/Th-17 cellular immune response (Decker and Edwards, 2021; Saso et al. 2021).
Since wP vaccines seem to prevent colonization in animal models, they may induce an immune response that promotes and/or acts more effectively on the surface of the mucosa compared to aP vaccines. These mechanisms are partially shaped by the choice of adjuvant in any vaccine and may be a consequence of the highly immunostimulatory LOS, which acts as an adjuvant by activating innate immune cells, for example, via Toll-like receptor (TLR)-4. In contrast, aP vaccines primarily contain a less reactive adjuvant, alum, which potentially contributes to the limited duration of effective pertussis immunity. Increasingly, cell-mediated immunity is considered to play a crucial role in vaccine efficacy and long-term protection via effector mechanisms. Protection after vaccination persists even in the absence of protective antibodies and is mediated by cellular immunity. New evidence from studies shows that local T memory cells accumulating in respiratory tissue after infection may be crucial for long-term immunity (Saso et al. 2021).
Comparisons between vaccines are complicated by the different compositions (and induced immune responses) and heterogeneity of aP vaccine formulations, immunization strategies, and schedules of administration. Due to the use of purified antigens in pertussis vaccines, the PT response to primary and booster immunization with an aP pertussis vaccine is usually more pronounced than the response after immunization with a wP vaccine. In comparison with wP vaccine, significantly higher anti-PT and anti-FHA responses have been reported with aP vaccines containing these antigens. The response to an aP vaccine was independent of the pre-immunization antibody titer, while the response to a wP vaccine strongly depended on the pre-immunization titer (Guiso et al. 2020; Saso et al. 2021).
The current use of aluminium adjuvants in aP vaccines has suboptimal effectiveness, as reflected in their inability to activate Th1-Th17 immune responses and memory T cells, which explains their insufficient ability to provide long-lasting immunity (Kilgore et al. 2016; Decker and Edwards, 2021; Saadatian-Elahi et al. 2016). Possible solutions include adding another non-aluminium adjuvant to existing aP vaccines used for school and adolescent booster doses or developing new acellular vaccines that induce Th1-Th17-Tfh cellular immune responses with appropriate adjuvants (Melvin et al. 2024). To improve the vaccine’s effectiveness, adding a TLR4 ligand as an adjuvant has been suggested as a possible strategy to enhance the immune response. Another solution is to develop new acellular pertussis vaccines with multiple components or whole-cell pertussis vaccines with detoxified LPS, which could make the vaccine more effective and safer (Decker and Edwards, 2021).
The known efficacy of whole-cell vaccines, combined with the cost-effectiveness of this approach, could be of greater benefit to people than more expensive vaccines consisting of purified proteins. It is important to remember that the development and approval of new vaccines is a lengthy process. Considering recent findings regarding the lack of protection against colonization or transmission by aP vaccination, maximizing the effectiveness of existing vaccines through prenatal vaccination, additional boosting, and alternative strategies is imperative (Saso et al. 2021).
For example, BPZE1, a live attenuated intranasal pertussis vaccine, induce immunity of the nasal mucosa and produces functional serum responses. BPZE1 has the potential to prevent B. pertussis infections, ultimately leading to reduced transmission and epidemic cycle reduction. Phase 2b clinical trials have been conducted, and it is planned to expand into phase 3 clinical trials (Lin et al. 2020).
- Future research
Despite high rates of immunization recent pertussis epidemics have occurred in the United States, Europe, Australia, and Japan. Epidemics seem inevitable in developed countries worldwide. Moreover, regardless of socioeconomic status, the highest mortality rates occur in newborns, who are also the most challenging population to treat and protect (Melvin et al. 2024). An additional factor in epidemics is the fact that pertussis vaccines do not provide lifelong immunity (Willey and Wood, 2019).
Fortunately, new tools from multiple disciplines are now available to us, providing a rich arsenal to guard against the risk of global complacency in the control of pertussis. Vaccines with improved adverse event profiles that provide higher efficacy will protect vulnerable populations, including neonates, very young infants, and older adults. Also, the identification of novel adjuvants to use with existing or new pertussis vaccines has the potential to augment immune responses, including cell-mediated immunity, across a wide range of ages. Strengthened surveillance for pertussis cases among vaccinees and non-vaccinees will help shed light on the emergence and distribution of vaccine-induced escape strains of B. pertussis that may emerge in different parts of the world.
Further research is also needed to understand changes in pertussis epidemiology that may result from changes in the mucosal environment, biofilms, and bacterial colonization in the nasopharynx and other mucosal sites induced by other vaccines. Also. Finally, the evaluation of dynamic models will help identify conditions under which a pertussis reemergence might be predicted and will aid in evaluating the potential role of booster doses, novel vaccines for neonates and other ages, and newly adjuvanted vaccines to prevent pertussis reemergence.
5. Conclusions
Despite high vaccination rates in many countries, cases and deaths from pertussis still occur among children and adults. The current surveillance systems are challenged by under-reporting, particularly among adolescents and adults, due to several factors such as low awareness among healthcare professionals, lack of standardized diagnostic tests, and weak surveillance infrastructure in many countries. Neither the currently available vaccines nor previous B. pertussis infection can provide long-term protection against later infection.
Developing new vaccines or enhancing the current ones will require multipronged approaches and global initiatives through public-private partnerships. The advancement of laboratory research in genomics, immunology, and bioinformatics is promising for a better understanding of the evolution of pertussis virulence and immunity that could lead to new opportunities for treatment and prevention of pertussis across all ages.
Funding: This research received no external funding.
Informed Consent Statement: Not applicable.
Data Availability Statement: Not applicable.
Acknowledgments: None.
Conflicts of Interest: The authors declare no conflicts of interest.
References:
Barkoff, A. M., Mertsola, J., Pierard, D., et al. (2018). Surveillance of circulating Bordetella pertussis strains in Europe during 1998 to 2015. Journal of Clinical Microbiology, 56(5). https://doi.org/10.1128/JCM.01998-17
Decker, M. D., & Edwards, K. M. (2021). Pertussis (Whooping Cough). Journal of Infectious Diseases, 224. https://doi.org/10.1093/infdis/jiaa469
Edwards, K. M., Decker, M. D., & Damron, F. H. (2023). Pertussis Vaccines. In Plotkin’s Vaccines (763-815.e19). Elsevier. https://doi.org/10.1016/B978-0-323-79058-1.00045-1
European Centre for Disease Prevention and Control (ECDC). (2022). ECDC Technical Report: Diagnosis and Molecular of Bordetella pertussis. ECDC.
Gradski zavod za javno zdravlje Beograd. (2024). Informacija o aktuelnoj epidemiji velikog kaslja na teritoriji Beograda 18-2-2024.
Guiso, N., Meade, B. D., & Wirsing von König, C. H. (2020). Pertussis vaccines: The first hundred years. Vaccine, 38(5). https://doi.org/10.1016/j.vaccine.2019.11.022
Institut za javno zdravlje Srbije Dr Milan Jovanovic Batut. (2024). Informacija o prijavljenim slučajevima pertusisa na teritoriji Republike Srbije.
Institut za javno zdravlje Srbije Dr Milan Jovanovic Batut. (2024). Izveštaj o epidemiološkoj situaciji pertusisa na teritoriji Republike Srbije u 2024. godini, zaključno sa 26.4.2024. godine.
Kilgore, P. E., Salim, A. M., Zervos, M. J., & Schmitt, H. J. (2016). Pertussis: Microbiology, disease, treatment, and prevention. Clinical Microbiology Reviews, 29(3). https://doi.org/10.1128/CMR.00083-15
Lin, A., Apostolovic, D., Jahnmatz, M., et al. (2020). Live attenuated pertussis vaccine BPZE1 induces a broad antibody response in humans. Journal of Clinical Investigation, 130(5). https://doi.org/10.1172/JCI135020
Melvin, J. A., Scheller, E. V., Miller, J. F., & Cotter, P. A. (2014). Bordetella pertussis pathogenesis: Current and future challenges. Nature Reviews Microbiology. https://doi.org/10.1038/nrmicro3235
Polak, M., Zasada, A. A., Mosiej, E., et al. (2019). Pertactin-deficient Bordetella pertussis isolates in Poland—a country with whole-cell pertussis primary vaccination. Microbes and Infection, 21(3-4). https://doi.org/10.1016/j.micinf.2018.12.001
Saadatian-Elahi, M., Aaby, P., Shann, F., et al. (2016). Heterologous vaccine effects. Vaccine, 34(34), 3923-3930. https://doi.org/10.1016/j.vaccine.2016.06.020
Saadatian-Elahi, M., Plotkin, S., Mills, K. H. G., et al. (2016). Pertussis: Biology, epidemiology and prevention. Vaccine, 34. https://doi.org/10.1016/j.vaccine.2016.10.029
Saso, A., Kampmann, B., & Roetynck, S. (2021). Vaccine-induced cellular immunity against Bordetella pertussis: Harnessing lessons from animal and human studies to improve design and testing of novel pertussis vaccines. Vaccines, 9(8). https://doi.org/10.3390/vaccines9080877
van der Zee, A., Schellekens, J. F. P., & Mooi, F. R. (2015). Laboratory diagnosis of pertussis. Clinical Microbiology Reviews, 28(4). https://doi.org/10.1128/CMR.00031-15
Willey, J., & Wood, D. (2019). Prescott’s Microbiology (11th ed., Vol. 39). McGraw Hill.
Wirsing von König, C. H. (2017). The immunological basis for immunization series: Module 4: Pertussis, update 2017.
World Health Organization. (2013). Annex 4: Recommendations to Assure the Quality, Safety and Efficacy of Acellular Pertussis Vaccines.