1. Introduction
Biocontrol represents the suppression of pathogenic species by introducing of their natural enemies and/or their secondary metabolites. In agriculture, these biological control agents (BCAs) can be used as substitutes for various chemical pesticides including bactericides and fungicides to obtain more eco-friendly plant health management methods that wouldn’t cause harm to the environment or human welfare. Biocontrol can also be used when pathogenic species develop resistance to conventional chemical agents or, for instance, when chemical agents are not available to control a particular plant disease (Fravel, 2005). Agriculturally important BCAs are commonly identified as representatives of the genera Bacillus and Pseudomonas. They produce secondary metabolites with great potential to combat a wide variety of phytopathogens. In addition, these metabolites have shown the capacity for growth promotion of plants associated with microorganisms synthesize them (Fravel, 2005; Manikandan et al. 2023). An overview of Bacillus spp. and Pseudomonas spp. as BCAs on plants is shown in Figure 1.
The main characteristics of BCAs are their high specificity to target plant pathogens, easy degradation, and low cost of mass production. Biocontrol microbes use different approaches to identify and neutralize their target, i.e., phytopathogens. These approaches include competition for resources, hyperparasitism, antibiosis and induction of systemic resistance in host plants (Živković et al. 2010). Biocontrol by competition for resources, whether for ecological niches or nutrients, is the limitation of pathogenic species fitness through the use of limited resources by biocontrol microbes that outcompete them (Hibbing et al. 2010). Hyperparasitism is the ability of biocontrol to reduce pathogen population by parasitizing them, while antibiotics or toxins
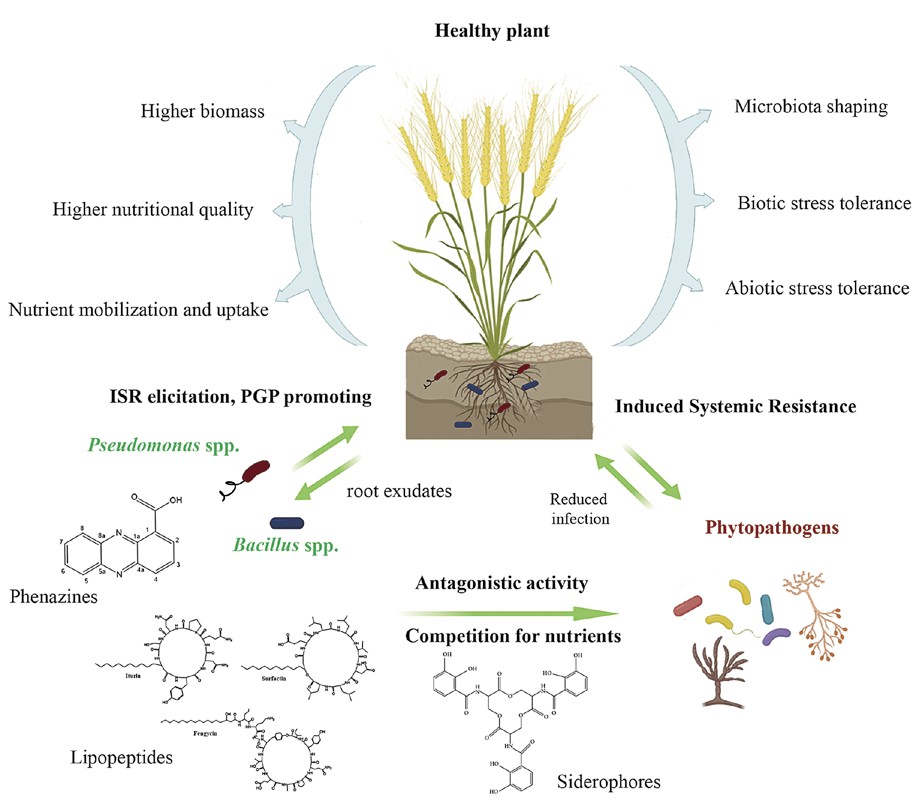
Figure 1. Plant growth-promoting and biocontrol mechanisms of beneficial Pseudomonas and Bacillus strains and their effects on plant health production generally mediates antibiosis by the antagonist, which can inhibit pathogen proliferation (Pankhurst & Lynch, 2005; Rani et al. 2020). Induction of systemic resistance by BCAs is the process by which they activate a plant’s physical or chemical barriers that protect it from phytopathogens. Induced resistance has been accomplished in plants through the colonization of roots by BCA strains. Once the community of BCA strains is established in the roots, plants are much more likely to resist colonization by pathogenic strains (Martinuz et al. 2012).
2. Pseudomonas species in biocontrol
Pseudomonas is a genus of non-spore-forming, Gram-negative, rod-shaped bacteria, found in a variety of habitats, including bulk soil and rhizosphere. Pseudomonas species use various substrates as nutrients and survive in different conditions that would be stressful for other bacteria. Therefore, they grow rapidly, adapt, and colonize different types of soil, including disease-suppressive soils (Weller, 2007). Considering ecological niches, Pseudomonas spp. is a highly successful colonizer in the rhizosphere, particularly in nutrient-poor soils. These soils have an effective excess of carbon because the plants are restricted in their growth by nutrient limitation and therefore cannot assimilate all available carbon (Lugtenberg et al. 2001; Tuomi et al. 1984). It has been determined that motility, production of the O-antigen of lipopolysaccharide (LPS), cellulose, thiamine, amino acids, and biotin, as well as the synthesis of an isoflavonoid-inducible efflux pump, are all essential points for effective colonization of the root and rhizosphere of the host plant (Lugtenberg et al. 2001). There is also evidence that Pseudomonas strains undergo genomic rearrangements in response to environmental changes that would allow better colonization of the rhizosphere (Martínez-Granero et al. 2005).
Many strains of Pseudomonasareknowntoproduce versatile metabolites with plant growth-promoting (PGP) properties and the ability to induce systemic resistance in plants. Some of these compounds include the enzyme 1-aminocyclopropane-1-car- boxylate (ACC) deaminase, which reduces ethylene levels in the root, thereby increasing root growth and length (Penrose & Glick, 2001); hormones such as indole acetic acid (IAA), abscisic acid (ABA), gibberellic acid (GA) and cytokinins (Dey et al. 2004; Dobbelaere et al. 2003; Patten & Glick, 2002); vitamins, namely niacin, pantothenic acid, thiamine, riboflavin and biotin (Revillas et al. 2000); cell-surface compounds (flagellin and lipopolysaccharides); and secondary metabolites such as lipopeptides, 2,4-diacetylphloroglucinol, siderophores, salicylic acid, pyochelin, pyocyanin, and volatile organic compounds (VOCs) (Audenaert et al. 2002; Park et al. 2015; Pieterse et al. 2014; Tran et al. 2007; Weller et al. 2012).
Considering the competition for nutrients, Pseudomonas species are very effective under iron- limiting conditions because they are able to synthesize iron-chelating compounds, i.e., siderophores. Since iron is present in proteins with [Fe-S] clusters and in heme, it is essential for different metabolic activities. One of the strategies for iron uptake is the binding of this micronutrient by iron-siderophore complexes, which have a high affinity for this molecule. This chelating and binding of iron by Pseudomonas spp. makes it less available to other microbes, including phytopathogens. Therefore, the synthesis of siderophores gives BCAs an important advantage in the competition for nutrients (Andrews et al. 2003). Among the high-affinity siderophores produced by fluorescent Pseudomonas spp. pyoverdines are highlighted as the main group. These green-yellow fluorescent pigments are synthesized by non-ribosomal peptide synthetases (Visca et al. 2007). The mechanism of iron uptake under aerobic conditions, mediated by iron-pyoverdine complexes, occurs in several steps. The non-fluorescent pyoverdine precursor ferribactin is synthesized in the cytoplasm and transported via the ABC transporter PvdE to the periplasm, where chromatophore maturation occurs. After maturation, pyoverdine is exported to the extracellular matrix where it comes into contact with Fe3+ creating ferripyoverdine. Further, ferripyoverdine is taken up via the FpvA receptor, which is a large porin with 22 β-strands that form a gated β-barrel called the TonB-dependent outer membrane receptor. Because the N-terminal domain of the protein constricts the pore, the N-terminal end of the receptor contains a domain called the TonB box. This domain interacts with TonB, an inner membrane protein that, together with ExbB and ExbD, transfers the energy of the proton motive force to allow opening of the gate and transport of the ferric complex into the periplasm. In the periplasm, an iron ion is reduced and released, followed by recycling and excretion of apo-pyoverdine. Recycling and excretion of pyoverdine involves the efflux pump, which consists of three proteins, PvdT, PvdR, and OpmQ. While the ferripyoverdine-binding protein is common in other species, it was not observed in the periplasm of Pseudomonas strains. This implies that iron alone is transported into the cytoplasm (Andrews et al. 2003; Imperi et al. 2009; Visca et al. 2007). In addition to pyoverdine as the primary siderophore, almost all fluorescent Pseudomonas species produce secondary siderophores with low affinity that are structurally diverse. Some may be derived from peptides and they are synthesized by non-ribosomal peptide synthetases, such as pyochelin, pseudomonine, yersiniabactin, corrugatin, and ornicorrugatins, whereas achromobactin, quinolobactin, and thioquinolobactin are synthesized differently (Cornelis, 2010; Matthijs et al. 2007). It’s estimated that these secondary siderophores are produced for energy conservation in situations where iron limitation is less drastic because the number of genes included for their biosynthesis is less than the number of genes for the synthesis of pyoverdine (Moon et al. 2008). Moreover, these siderophores have shown catalytic properties in some antibiosis mechanisms (Matthijs et al. 2007). An example of siderophore- mediated control of phytopathogens is the ability of Pseudomonas syringae pv. syringae to inhibit the growth of P. syringae pv. glycinea, Gram-negative bacterium that causes bacterial blight (Wensing et al. 2010).
MembersofthegenusPseudomonasproduceawide range of antimicrobial compounds with the potential to inhibit the growth of different phytopathogens. Some of these antimicrobials includes pyrazines, phenazine-1-carboxylic acid (PCA), phenazine-1- carboxamide, 2,4-diacetylphloroglucinol, pyrrol- nitrin, hydrogen cyanide (HCN), pyoluteorin and protein-type compounds (bacteriocins). Studies have shown that cyclic lipopeptides produced by Pseudomonas species, i.e., nunamycin, nunapeptin, brasmycin, and braspeptin are essential for antifungal activity, while sessilins and orfamides displayed additive roles in the suppressing some fungal diseases (Dimkić et al. 2022). Phenazine-1- carboxylic acid together with 4-acetamidobenzoic acid, 4-hydroxybenzoic acid, and 2-hydroxyphenyl nitrite extracted from Pseudomonas synxantha showed antifungal activity against Monilinia laxa, an ascomycete fungus responsible for brown rot disease infecting many different types of stone fruit (Janakiev et al. 2019). The same compound found in Pseudomonas orientalis X2-1P showed antibacterial properties against Xanthomonas campestris pv. campestris, a pathogenic bacterium that affecting the growth and quality of various cultivated Brassicaceae (Jelušić et al. 2021), while PCA from Pseudomonas fluorescens 2-79 shows protective and PGP properties against wheat infected by Gaeumannomyces graminis var. tritici (Thomashow & Weller, 1988). Pyocyanin is a toxic PCA-derived metabolite produced by P. aeruginosa that promotes the production of reactive oxygen species. It has antibacterial properties that increase fitness in competition with other bacterial species, as well as antifungal abilities against the oomycetes P. myriotylum and Pythium spp. and the filamentous fungi Septoria tritici and Fusarium spp. (Anjaiah et al. 1998; Flaishman et al. 1990; Tambong & Höfte, 2001).
Another important group of antimicrobials are VOCs, small molecules synthesized by primary and secondary metabolic pathways that include chemical classes such as alcohols, esters, aliphatic and aromatic hydrocarbons, terpenes, nitrogen, and sulfur compounds, and others. VOCs such as nonanal, benzothiazole, and 2-ethyl-1-hexanol, found in the bacterium Pseudomonas chlororaphis PA23, together with non-volatile antibiotics phenazine and pyrrolnitrin, are able to protect the phyllosphere of canola from the plant pathogenic fungus Sclerotinia sclerotiorum, which causes white mold disease (Fernando et al. 2007). VOCs such as 2,5-dimethylpyrazine, 2-methylpyrazine, 2-ethyl-5-methylpyrazine, 2-ethyl 3, 6-dimethylpyrazine and dimethyl trisulfide from Pseudomonas putida BP25 have antifungal activity against phytopathogenic fungi Phytophthora capsici, Pythium myriotylum, Colletotrichum gloeosporioides, Rhizoctonia solani, Gibberella moniliformis, Athelia rolfsii, Magnaporthe oryzae, the bacterium Ralstonia pseudosolanacearum, and the plant-parasitic nematode Radopholus similis (Agisha et al. 2019).
Biocontrol mechanisms of Pseudomonas strains also include the production and extraction of HCN, a secondary metabolite that has antimicrobial properties. It has been shown that the bacterial strain fluorescens CHA0 can suppress black root rot of tobacco, a disease caused by the fungus Thielaviopsis basicola, by synthesising HCN in cooperation with other antifungal secondary metabolites (Voisard et al. 1989). Antifungal properties are also exhibited by the compound 2,4-diacetylphloroglucinol, which is extracted from different fluorescent Pseudomonas species, and can inhibit the growth of various fungal species (Weller et al. 2002).
3. Bacillus species in biocontrol
Bacillus is a genus of spore-forming, Gram- positive, rod-shaped bacteria with a very diverse secondary metabolism that allows the production of a wide range of structurally diverse compounds. For example, on average, about 4-5% of the whole genomes of Bacillus subtilis strains is dedicated to the synthesis of secondary metabolites (Stein, 2005). These compounds often have PGP properties that can manifest through direct or indirect mechanisms. Direct mechanisms include phytostimulating compounds that provide an advantage in competition for nutrients and ecological niches. Indirect approaches, on the other hand, include the synthesis and use of compounds, such as antimicrobials, that have an inhibitory effect on the pathogenic organism (Ahmad et al. 2008). Among phytostimulating compounds, plant hormones are of particular interest, as bacteria of the genus Bacillus have been found to be able to produce cytokinins, auxins, gibberellins, and abscisic acid, which positively influence plant growth and development (Arkhipova et al. 2007; Idriss et al. 2002; Joo et al. 2004; Xu et al. 2018). Other important phytostimulants include VOCs such as 2,3-butanediol and acetoin, which have been shown to be essential metabolites in chemical signal transduction between B. strains and Arabidopsis thaliana (Ryu et al. 2003). Although other volatile compounds, aldehydes, ketones, alcohols, 1-octen- 3-ol and butyrolactone, have also been detected in chemical signaling, their role in plant development and plant-bacterial interactions has not been fully elucidate (Gutiérrez-Luna et al. 2010).
Bacillus strains are known for their ability to produce various antimicrobials, of which lipopeptides are the most important class. Their molecular structure is predominantly rigid, hydrophobic, and cyclic, and consists of D-amino acids with high resistance to hydrolysis by peptidases and proteases. These antimicrobials are also resistant to oxidation thanks to their already oxidized structure and thioether bonds (Katz & Demain, 1977). There are two different pathways of lipopeptide biosynthesis. The first pathway is the non-ribosomal synthesis of peptides by large megaenzymes (NRPSs), while the second pathway is the ribosomal synthesis of linear precursor peptides that undergo post-translational modification and proteolytic processing (Stein, 2005).
Ribosomally synthesized antimicrobial lipopep- tides are mainly small, heat-stable, amphiphilic pro- teins marked as bacteriocins. These compounds are produced by strains of specific microbial species and are effective against other closely related bacteria be- cause their spectrum of inhibition is usually limited and their mechanism of action involves interaction with the wall of target cell (Field et al. 2007). Bacillus spp. bacteriocins can be classified into three groups. Class I includes peptides that undergo post-trans- lational modification. The first three subclasses are considered lantibiotics because they are modified with lanthionine and b-metyl lanthionine; the fourth subclass has other posttranslational modifications. Based on their structure, two types of lanthibiotics are described. Type A lanthibiotics, which have a linear structure, are able to form voltage-dependent pores in the cytoplasmic membrane of Gram-posi- tive target cells and destroy them. Gram-positive lanthibiotic producers have special mechanisms of self-protection against their products. Immunity is based on export of the lantibiotic from the cytoplas- mic membrane to the extracellular space, which oc- curs through the transporter proteins LanFEG, or on the membrane-bound lipoproteins LanI, which pre- vent lantibiotics from forming a lipid pore II and also prevent high local concentrations of the lantibiotic near the cytoplasmic membrane (Stein et al. 2005; Stein et al. 2003).
The bacteriocins of the class II belong to the small peptides synthesized ribosomally but not modified, which are heat stable. Large proteins with antibacterial activities based on their enzymatic activities are placed into class III. This group also includes peptides and proteins with antimicrobial properties that are not sufficiently defined to classify them, so they are generally referred to as bacteriocin- like inhibitory substances (BLIS) (Lodemann et al. 2008). Due to their limited inhibitory spectrum, Bacillus strains producing bacteriocin do not play a major role in biocontrol. However, non-ribosomally synthesized lipopeptides and peptides, such as fengycins, iturins, surfactins, and kurstakins have shown great potential to control a variety of plant pathogens (Dimkić et al. 2013; Dimkić et al. 2017; Mishra et al. 2015).
Non-ribosomally synthesized peptides and lipo- peptides are synthesized by non-ribosomal peptide synthetases (NRPSs), large multi-domain enzyme complexes with modular structure. They are a large, diverse group of compounds, including amino acids, and amino- or hydroxyl-fatty acids with hydrocarbon chains of varying lengths. They can be additionally modified by acylation, methylation, or glycosylation. The modular structure of megaenzymes is crucial for the process of amino-acid incorporation, as each module has several domains, including PCP (pepti- dyl carrier protein) and domain A, which is respon- sible for adenylation and thus activation of an amino acid (Fischbach & Walsh, 2006). The process of bio- synthesis begins with the transfer of the phosphop- antetheinyl group to the PCP (peptidyl carrier pro- tein), catalyzed by the specific phosphopantetheinyl transferase and the activation of the amino acid. The activated amino acid is then transferred to the 4ʹ- phosphopantetheine group of the PCP, resulting in the formation of a thioester bond. The C domain of a module is responsible for catalyzing the forma- tion of a peptide bond between amino acids during polymerization. In addition, the E domain catalyzes the epimerization of certain amino acids since many non-ribosomal peptides contain D- and L- stereo- isomers of amino acids. This property contributes to its resistance to the action of proteolytic enzymes (Fischbach & Walsh, 2006). Non-ribosomally syn- thesized antimicrobial lipopeptides from Bacillus species can be divided into four prominent families – iturins, surfactins, fengycins, and kurstakins. These cyclic lipopeptides consist of seven or ten amino acid residues linked to a fatty acid derivative. Due to the different lengths of the fatty acid hydrocarbon chains or the different composition of the amino acids, these molecules can assume many isomeric forms (Ongena & Jacques, 2008).
Lipopeptides from the iturin family are circular heptapeptides attached to a β-amino fatty acid chain with a length of 14 to 17 carbon atoms and exhibit high in vitro antifungal activity against various yeast and fungal strains but limited antibacterial and no antiviral activities. Iturin A and C, bacillomycin D, F, L, and LC and mycosubtilin are the seven major variants within the iturin family (Jacques, 2011; Ongena & Jacques, 2008). Iturin A, produced by various strains of B. subtilis, B. amyloliquefaciens and other Bacillus species, has plant-protective activity against many fungi such as Phomopsis sclerotioides, Fusarium oxysporum f. sp. radicis-lycopersici, Rosellinia necatrix, Gloeosporium gloeosporioides, Alternaria mali, Botrytis elliptica, Botrytis cinerea, Colletotrichum musae, Sclerotium rolfsii, Glomerella cingulata, R. solani, Alternaria citri, C. gloeosporioides, Penicillium crustosum, F. graminearum and Pythium irregulare (Arrebola et al. 2010; Cazorla et al. 2007; Cho et al. 2003; Hsieh et al. 2008; Kita et al. 2005; Zhao et al. 2014). Antibacterial activity manifested in suppression of X. campestris pv. campestris, growth has been demonstrated with a compound known as iturin A2 produced by B. amyloliquefaciens. In addition, iturins produced by B. subtilis suppress diseases caused by X. campestris pv. cucurbitae and Pectobacterium carotovorum subsp. carotovorum (Falardeau et al. 2013; Yoshida et al. 2001). Two bacillomycin D analogs produced by B. subtilis AU195 showed antifungal properties against Aspergillus flavus, while B. amyloliquefaciens exhibited potent antifungal activities against R. solani due to the production of bacillomycin L as well as bacillomycin D (Chowdhury et al. 2015; Li et al. 2014; Moyne et al. 2001). The fungicidal abilities of bacillomycin D were also confirmed against F. graminearum, Alternaria alternata, Cryphonectria parasitica, and P. capsici (Zhao et al. 2010). Mycosubtilin overproduced by B. subtilis strain BBG100 significantly reduced fungal infection of tomato seedlings caused by Pythium aphanidermatum, while overproduction of the same iturin by B. subtilis strain BBG125 showed antifungal activity against B. cinerea and F. oxysporum (Béchet et al. 2013; Leclère et al. 2005).
The surfactin family consists of amphiphilic cyclic peptides composed of 7 α-amino acids attached to a single β-hydroxy fatty acid of 13 to 16 carbons in length (Jacques, 2011). These biosurfactants are compounds produced by microbial cells, distributed over their surface, or excreted to reduce surface and interfacial tension while contributing to cell motility, adhesion to biofilm formation, and plant immune responses (Costa et al. 2018; Fira et al. 2018; Jourdan et al. 2009). Different variants of surfactin, pumilacidin, lychenisin, and halobacilin belong to this family. In addition to the aforementioned properties, they are also known for their ability to induce irreversible pore and ion channel formation in cell membranes of various bacteria, viruses, and fungi, thereby disrupting and destabilizing them (Ongena & Jacques, 2008). The mechanism of membrane disruption begins with surfactants binding to the outer membrane of the bacterial cell envelope and penetrating the bacterial cell wall before interacting with the inner phospholipid membrane. It has been found that size, charge, molecular architecture, critical micellar concentration, aggregation numbers, chain length, and degree of saturation are all properties of surfactants that strongly influence their binding efficacy. On the other hand, whether surfactants dissolve the outer membrane or penetrate the phospholipid structure of the inner membrane through channels remains an open question (Sharma et al. 2022). Once they reach the membrane, dimerized surfactins insert into lipid bilayers, helate mono- and divalent cations, and alter cell membrane permeability either through channel formation or detergent-like membrane solubilization (Fracchia et al. 2012; Sharma et al. 2022). Surfactins, especially surfactin and pumilacidin, produced by various strains of B. subtilis, B. amyloliquefaciens and other Bacillus species showed considerable antimicrobial activity against many plant pathogens, such as S. sclerotiorum, R. solani, Fusarium solani, X. axonopodis pv. glycines, Aspergillus flavus, C. gloeosporioides, P. aphanidermatum, S. rolfsii, M. grisea, Curvularia lunata, Rhizoctonia bataticola and Fusarium verticillioides (Li et al. 2014; Melo et al. 2009; Mohammadipour et al. 2009; Preecha et al. 2010; Snook et al. 2009; Tendulkar et al. 2007). In one of the previous studies, mass spectrometry analysis confirmed the presence of surfactin in the ethyl acetate extract of B. amyloliquefaciens strain SS-12.6, which showed strong growth inhibitory properties against a wide range of postharvest fungal pathogens, in vitro and in situ, on apple fruit as well as against all tested strains of X. arboricola pv. juglandis from walnut fruit (Dimkić et al. 2013).
Lipopeptides from the fengycin family are amphiphilic cyclic peptides composed of 10 α-amino acids attached to a β-hydroxy fatty acid of 14 to 18 carbons in length (Jacques, 2011). These compounds were extracted mainly from B. subtilis and also act on the target cells by interacting with the cell membrane and changing its structure and permeability (Deleu et al. 2008; Fira et al. 2018). Fengycins, highly recognized for their antimicrobial properties, have been the subject of numerous studies demonstrating their growth inhibitory activities against fungi such as F. oxysporum, Fusarium graminearum, Fusarium culmorum, Fusarium moniliforme, Mycosphaerella fijiensis, B. cinerea, M. laxa/fructicola, R. solani, Verticillium dahliae, F. solani, Phytophthora parasitica, and C. gloeosporioides (Chan et al. 2009; Falardeau et al. 2013; Rebib et al. 2012; Villegas-Escobar et al. 2013; Yánez-Mendizábal et al. 2012).
Kurstakins are lipoheptapeptides produced primarily by Bacillus thuringiensis but also found in other species of the genus Bacillus, such as B. cereus. It has been discovered that their antimicrobial activity is also due to their ability to form pores in cell membranes, but their activity is recognized as species- specific. Stachybotrys chartarum is one of the fungi sensitive to kurstakins produced by B. thuringiensis (Béchet et al. 2012). Many studies have shown that different lipopeptide antimicrobial compounds often cooperate with the common goal of suppressing the activity of phytopathogens. For example, antifungal activity against the fungus Pestalotiopsis eugeniae was activated when both iturin A and surfactin produced by B. subtilis BS-99 were present (Lin et al. 2011). Moreover, the cooperation between lipopeptides did not to prove to be a unique case, as compounds produced by antagonistic Bacillus strains in synergy with savory oil or when thyme and savory oils were applied simultaneously in situ to marigold seeds, also showed positive effects on reducing overall fungal infection without adverse effects on seed germination (Dimkić et al. 2015).
Bacillus species can act via the induction of systemic resistance in plant hosts by producing various volatiles, such as alcohols, aldehydes, aromatics, sulfides and ketones. By activating jasmonic acid, salicylic acid or ethylene signaling pathways, compounds from Bacillus species (e.g., lipopeptides, polyketides, and volatiles) stimulate the expression of genes encoding pathogenesis-related proteins and other defense-related proteins in plant hosts. These compounds have also been shown to be able to inhibit quorum sensing in competing bacteria as well as inhibit the expression of genes involved in mycelial growth, penetration, sporulation, and virulence of fungal pathogens (Dimkić et al. 2022).
4. Conclusions
In view of the studies mentioned so far, the metabolites of Pseudomonas spp. and Bacillus spp. have immense biological control potential against a wide range of agronomically important fungal and bacterial phytopathogens. Thus, instead of applying already overproduced chemical fertilizers and pesticides, the use of Pseudomonas and Bacillus strains in the form of bioformulations can achieve the same, if not better, results in protecting plant health while maintaining ecosystem health. At the same time, lower production costs make BCAs much more affordable for many farmers in developing countries, which is another step toward sustainable agriculture.
Funding: This research was funded by the Ministry of Education, Science and Technological developments of the Republic of Serbia, Grant No.: 451-03-47/2023-01/200178 and by the Collaborative Research Programme (CRP) – ICGEB Research Grants Programme [Contracts No. CRP/SRB19-02]. Informed Consent Statement: Not applicable
Data Availability Statement: Not applicable
Acknowledgments: In loving memory of Prof. dr Djordje Fira, a great scientist and friend – your presence we miss, your memories we treasure Conflicts of Interest: The authors declare no conflicts of interest.
References
- Agisha, V. , Kumar, A., Eapen, S. J., Sheoran, N., & Suseelabhai, R. (2019). Broad-spectrum antimicrobial activity of volatile organic compounds from endophytic Pseudomonas putida BP25 against diverse plant pathogens. Biocontrol Science and Technology, 29(11), 1069-1089.
- Ahmad, F., Ahmad, , & Khan, M. (2008). Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiological Research, 163(2), 173-181.
- Andrews, C., Robinson, A. K., & Rodríguez-Quiño- nes, F. (2003). Bacterial iron homeostasis. FEMS Microbiology Reviews, 27(2-3), 215-237.
- Anjaiah, V., Koedam, , Nowak-Thompson, B., Loper, E., Höfte, M., Tambong, J. T., & Cornelis, P. (1998). Involvement of phenazines and anthranilate in the antagonism of Pseudomonas aeruginosa PNA1 and Tn5 derivatives toward Fusarium spp. and Pythium spp. Molecular Plant-Microbe Interactions, 11(9), 847-854.
- Arkhipova, T., Prinsen, , Veselov, S., Martinenko, E., Melentiev, A., & Kudoyarova, G. (2007). Cytokinin producing bacteria enhance plant growth in drying soil. Plant and Soil, 292(1), 305-315.
- Arrebola, , Jacobs, R., & Korsten, L. (2010). Iturin A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. Journal of Applied Microbiology, 108(2), 386-395.
- Audenaert, , Pattery, T., Cornelis, P., & Höfte, M. (2002). Induction of Systemic Resistance to Botrytis cinerea in Tomato by Pseudomonas aeruginosa 7NSK2: Role of Salicylic Acid, Pyochelin, and Pyocyanin. Molecular Plant-Microbe Interactions®, 15(11), 1147-1156.
- Béchet, , Caradec, T., Hussein, W., Abderrahmani, A., Chollet, M., Leclère, V., et al. (2012). Structure, biosynthesis, and properties of kurstakins, nonribosomal lipopeptides from Bacillus spp. Applied Microbiology and Biotechnology, 95(3), 593-600.
- Béchet, , Castéra-Guy, J., Guez, J. S., Chihib, N.-E., Coucheney, F., Coutte, F., et al. (2013). Production of a novel mixture of mycosubtilins by mutants of Bacillus subtilis. Bioresource Technology, 145, 264-270.
- Cazorla, F., Romero, D., Pérez‐García, , Lugtenberg, B., Vicente, A. D., & Bloemberg, G. (2007). Isolation and characterization of antagonistic Bacillus subtilis strains from the avocado rhizoplane displaying biocontrol activity. Journal of Applied Microbiology, 103(5), 1950-1959.
- Chan, Y. , Savard, M. E., Reid, L. M., Cyr, T., McCormick, W. A., & Seguin, C. (2009). Identification of lipopeptide antibiotics of a Bacillus subtilis isolate and their control of Fusarium graminearum diseases in maize and wheat. BioControl, 54(4), 567-574.
- Cho, J., Lee, S. K., Cha, B. J., Kim, Y. H., & Shin,S. (2003). Detection and characterization of the Gloeosporium gloeosporioides growth inhibitory compound iturin A from Bacillus subtilis strain KS03. FEMS Microbiology Letters, 223(1), 47-51.
- Chowdhury, P., Hartmann, A., Gao, X., & Borriss, R. (2015). Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42–a review. Frontiers in Microbiology, 6, 780.
- Cornelis, P. (2010). Iron uptake and metabolism in pseudomonad Applied Microbiology and Biotechnology, 86(6), 1637-1645.
- Costa, J. V., Treichel, H., Santos, L. O., & Martins, V. G. (2018). Chapter 16 – Solid-State Fermentation for the Production of Biosurfactants and Their Applications. In A. Pandey, C. Larroche, & C. R. Soccol (Eds.), Current Developments in Biotechnology and Bioengineering (pp. 357-372). Amsterdam, The Netherlands: Elsevier.
- Deleu, , Paquot, M., & Nylander, T. (2008). Effect of fengycin, a lipopeptide produced by Bacillus subtilis, on model biomembranes. Biophysical Journal, 94(7), 2667-2679.
- Dey, R., Pal, K., Bhatt, D. M., & Chauhan, S. M. (2004). Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth-promoting rhizobacteria. Microbiological Research, 159(4), 371-394.
- Dimkić, , Živković, S., Berić, T., Ivanović, Ž., Gavrilović, V., Stanković, S., & Fira, D. (2013). Characterization and evaluation of two Bacillus strains, SS-12.6 and SS-13.1, as potential agents for the control of phytopathogenic bacteria and fungi. Biological Control, 65(3), 312-321.
- Dimkić, , Berić, T., Stević, T., Pavlović, S., Šavikin, K., Fira, D., & Stanković, S. (2015). Additive and synergistic effects of Bacillus spp. isolates and essential oils on the control of phytopathogenic and saprophytic fungi from medicinal plants and marigold seeds. Biological Control, 87, 6-13.
- Dimkić, , Stanković, S., Nišavić, M., Petković, M., Ristivojević, P., Fira, D., & Berić, T. (2017). The profile and antimicrobial activity of Bacillus lipopeptide extracts of five potential biocontrol strains. Frontiers in Microbiology, 8, 925.
- Dimkić, I., Janakiev, T., Petrović, M., Degrassi, G., & Fira, D. (2022). Plant-associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms-A review. Physiological and Molecular Plant Pathology, 117,
- Dobbelaere, , Vanderleyden, J., & Okon, Y. (2003). Plant Growth-Promoting Effects of Diazotrophs in the Rhizosphere. Critical Reviews in Plant Sciences, 22(2), 107-149.
- Falardeau, J., Wise, , Novitsky, L., & Avis, T. J. (2013). Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens. Journal of Chemical Ecology, 39(7), 869-878.
- Fernando, W., Nakkeeran, , Zhang, Y., & Savchuk, S. (2007). Biological control of Sclerotinia sclerotiorum (Lib.) de Bary by Pseudomonas and Bacillus species on canola petals. Crop protection, 26(2), 100-107.
- Field, D., Cotter, P., Hill, C., & Ross, R. P. (2007). Bacteriocin biosynthesis, structure, and functio Research and Applications in Bacteriocins, 4, 5-41.
- Fira, D., Dimkić, , Berić, T., Lozo, J., & Stanković, S. (2018). Biological control of plant pathogens by Bacillus species. Journal of Biotechnology, 285, 44-55.
- Fischbach, A., & Walsh, C. T. (2006). Assembly- line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery, and mechanisms. Chemical Reviews, 106(8), 3468-3496.
- Flaishman, , Eyal, Z., Voisard, C., & Haas, D. (1990). Suppression of Septoria tritici by Phenazine- or Siderophore-deficient mutants of Pseudomonas. Current Microbiology, 20(2), 121-124.
- Fracchia, , Cavallo, M., Martinotti, M. G., & Banat, M. (2012). Biosurfactants and bioemulsifiers biomedical and related applications–present status and future potentials. Biomedical Science, Engineering and Technology, 14(1), 1-49.
- Fravel, D. R. (2005). Commercialization and Implementation of Biocontrol. Annual Review of Phytopathology, 43(1), 337-359.
- Gutiérrez-Luna, F. , López-Bucio, J., Altamirano- Hernández, J., Valencia-Cantero, E., de la Cruz, H. R., & Macías-Rodríguez, L. (2010). Plant growth- promoting rhizobacteria modulate root-system architecture in Arabidopsis thaliana through volatile organic compound emission. Symbiosis, 51(1), 75-83.
- Hibbing, E., Fuqua, C., Parsek, M. R., & Peterson, S. B. (2010). Bacterial competition: surviving and thriving in the microbial jungle. Nature Reviews Microbiology, 8(1), 15-25.
- Hsieh, F. , Lin, T.-C., Meng, M., & Kao, S. S. (2008). Comparing methods for identifying Bacillus strains capable of producing the antifungal lipopeptide iturin A. Current Microbiology, 56(1), 1-5.
- Idriss, E., Makarewicz, O., Farouk, A., Rosner, K., Greiner, R., Bochow, H., et al. (2002). Extracellular phytase activity of Bacillus amyloliquefaciens FZB45 contributes to its plant-growth-promoting effect. Microbiology, 148(7), 2097-2109.
- Imperi, F., Tiburzi, F., & Visca, P. (2009). Molecular basis of pyoverdine siderophore recycling in Pseudomonas aeruginosa. Proceedings of the National Academy of Sciences, 106(48), 20440-20445.
- Jacques, P. (2011). Surfactin and Other Lipopeptides from Bacillus spp. In Soberón-Chávez (Ed.), Biosurfactants: From Genes to Applications (pp. 57-91). Berlin, Heidelberg: Springer Berlin Heidelberg.
- Janakiev, T., Dimkić, , Unković, N., Ljaljević Grbić, M., Opsenica, D., Gašić, U., et al. (2019). Phyllosphere fungal communities of plum and antifungal activity of indigenous phenazine-producing Pseudomonas synxantha against Monilinia laxa. Frontiers in Microbiology, 10, 2287.
- Jelušić, , Popović, T., Dimkić, I., Mitrović, P., Peeters, K., Višnjevec, A. M., et al. (2021). Changes in the winter oilseed rape microbiome affected by Xanthomonas campestris pv. campestris and biocontrol potential of the indigenous Bacillus and Pseudomonas isolates. Biological Control, 160, 104695.
- Joo, G. J., Kim, Y. M., Lee, J., Song, K. S., & Rhee, I. K. (2004). Growth promotion of red pepper plug seedlings and the production of gibberellins by Bacillus cereus, Bacillus macroides and Bacillus pumilus. Biotechnology Letters, 26(6), 487-491.
- Jourdan, , Henry, G., Duby, F., Dommes, J., Barthelemy, J. P., Thonart, P., & Ongena, M. (2009). Insights into the defense-related events occurring in plant cells following perception of surfactin-type lipopeptide from Bacillus subtilis. Molecular Plant- Microbe Interactions, 22(4), 456-468.
- Katz, , & Demain, A. L. (1977). The peptide antibiotics of Bacillus: chemistry, biogenesis, and possible functions. Bacteriological Reviews, 41(2), 449-474.
- Kita, , Ohya, T., Uekusa, H., Nomura, K., Manago, M., & Shoda, M. (2005). Biological control of damping-off of tomato seedlings and cucumber Phomopsis root rot by Bacillus subtilis RB14-C. Japan Agricultural Research Quarterly: JARQ, 39(2), 109-114.
- Leclère, V., Béchet, , Adam, A., Guez, J.-S., Wathelet, B., Ongena, M., et al. (2005). Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Applied and Environmental Microbiology, 71(8), 4577-4584.
- Li, B., Li, Q., Xu, Z., Zhang, N., Shen, Q., & Zhang, R. (2014). Responses of beneficial Bacillus amyloliquefaciens SQR9 to different soilborne fungal pathogens through the alteration of antifungal compounds productio Frontiers in Microbiology, 5, 636.
- Lin, H. F., Chen, T. H., & Da Liu, S. (2011). The antifungal mechanism of Bacillus subtilis against Pestalotiopsis eugeniae infected to wax apple and development for commercial applicatio African Journal of Microbiology Research, 5(14).
- Lodemann, U., Lorenz, B. , Weyrauch, K. D., & Martens, H. (2008). Effects of Bacillus cereus var. toyoi as probiotic feed supplement on intestinal transport and barrier function in piglets. Archives of Animal Nutrition, 62(2), 87-106.
- Lugtenberg, B. J., Dekkers, L., & Bloemberg, G. V. (2001). Molecular determinants of rhizosphere colonization by Pseudomonas. Annual Review of Phytopathology, 39(1), 461-490.
- Manikandan, , Anandham, R., Johnson, I., Krishnamoorthy, R., Senthilkumar, M., Raghu, R., et al. (2023). Bacillus and Streptomyces for Management of Biotic Stresses in Plants for Sustainable Agriculture. In S. Chhabra, R. Prasad, N. R. Maddela, & N. Tuteja (Eds.), Plant Microbiome for Plant Productivity and Sustainable Agriculture (pp. 263-288). Singapore: Springer Nature Singapore.
- Martínez-Granero, F., Capdevila, , Sánchez- Contreras, M., Martín, M., & Rivilla, R. (2005). Two site-specific recombinases are implicated in phenotypic variation and competitive rhizosphere colonization in Pseudomonas fluorescens. Microbiology, 151(3), 975-983.
- Martinuz, A., Schouten, A., & Sikora, R. (2012). Systemically induced resistance and microbial competitive exclusion: implications on biological control. Phytopathology, 102(3), 260-266.
- Matthijs, , Tehrani, K. A., Laus, G., Jackson, R. W., Cooper, R. M., & Cornelis, P. (2007). Thioquinolobactin, a Pseudomonas siderophore with antifungal and anti‐ Pythium activity. Environmental Microbiology, 9(2), 425-434.
- Melo, F. P. d., Fiore, M. F., Moraes, L. A. B. d., Silva- Stenico, M. E., Scramin, S., Teixeira, M. d. A., & Melo, S. d. (2009). Antifungal compound produced by the cassava endophyte Bacillus pumilus MAIIIM4A. Scientia Agricola, 66, 583-592.
- Mishra, J., Tewari, , Singh, S., & Arora, N. K. (2015). Biopesticides: Where We Stand? In N. K. Arora (Ed.), Plant Microbes Symbiosis: Applied Facets (pp. 37-75). New Delhi: Springer India.
- Mohammadipour, , Mousivand, M., Salehi Jouzani, G., & Abbasalizadeh, S. (2009). Molecular and biochemical characterization of Iranian surfactin- producing Bacillus subtilis isolates and evaluation of their biocontrol potential against Aspergillus flavus and Colletotrichum gloeosporioides. Canadian Journal of Microbiology, 55(4), 395-404.
- Moon, D., Zhang, X. X., Matthijs, S., Schäfer, M., Budzikiewicz, H., & Rainey, P. B. (2008). Genomic, genetic and structural analysis of pyoverdine-mediated iron acquisition in the plant growth-promoting bacterium Pseudomonas fluorescens SBW25. BMC Microbiology, 8(1), 1-13.
- Moyne, L., Shelby, R., Cleveland, T., & Tuzun, S. (2001). Bacillomycin D: an iturin with antifungal activity against Aspergillus flavus. Journal of Applied Microbiology, 90(4), 622-629.
- Ongena, , & Jacques, P. (2008). Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends in Microbiology, 16(3), 115-125.
- Pankhurst, E., & Lynch, J. M. (2005). Biocontrol of soil-borne plant diseases In D. Hillel (Ed.), Encyclopedia of Soils in the Environment (pp. 129-136). Oxford: Elsevier.
- Park, Y. S., Dutta, S., Ann, M., Raaijmakers, J. M., & Park, K. (2015). Promotion of plant growth by Pseudomonas fluorescens strain SS101 via novel volatile organic compound Biochemical and Biophysical Research Communications, 461(2), 361-365.
- Patten, L., & Glick, B. R. (2002). Regulation of indoleacetic acid production in Pseudomonas putida GR12-2 by tryptophan and the stationary-phase sigma factor RpoS. Canadian Journal of Microbiology, 48(7), 635-642.
- Penrose, D. , & Glick, B. R. (2001). Levels of ACC and related compounds in exudate and extracts of canola seeds treated with ACC deaminase-containing plant growth-promoting bacteria. Canadian Journal of Microbiology, 47(4), 368-372.
- Pieterse, M. J., Zamioudis, C., Berendsen, R. L., Weller, D. M., Wees, S. C. M. V., & Bakker, P. A. H. M. (2014). Induced Systemic Resistance by Beneficial Microbes. Annual Review of Phytopathology, 52(1), 347-375.
- Preecha, , Sadowsky, M. J., & Prathuangwong, S. (2010). Lipopeptide surfactin produced by Bacillus amyloliquefaciens KPS46 is required for biocontrol efficacy against Xanthomonas axonopodis pv. glycines. Agriculture and Natural Resources, 44(1), 84-99.
- Rani, , Tyagi, K., & Jha, G. (2020). Chapter 10 – Advancements in plant disease control strategies. In N. Tuteja, R. Tuteja, N. Passricha, & S. K. Saifi (Eds.), Advancement in Crop Improvement Techniques (pp. 141-157). Sawston, Cambridge: Woodhead Publishing.
- Rebib, , Hedi, A., Rousset, M., Boudabous, A., Limam, F., & Sadfi-Zouaoui, N. (2012). Biological control of Fusarium foot rot of wheat using fengycin- producing Bacillus subtilis isolated from salty soil. African Journal of Biotechnology, 11(34), 8464-8475.
- Revillas, J. J., Rodelas, B., Pozo, , Martínez‐Toledo, M. V., & González‐López, J. (2000). Production of B‐group vitamins by two Azotobacter strains with phenolic compounds as sole carbon source under diazotrophic and adiazotrophic conditions. Journal of Applied Microbiology, 89(3), 486-493.
- Ryu, M., Farag, M. A., Hu, C. H., Reddy, M. S., Wei, H. X., Paré, P. W., & Kloepper, J. W. (2003). Bacterial volatiles promote growth in Arabidopsis. Proceedings of the National Academy of Sciences, 100(8), 4927-4932.
- Sharma, P., Vaiwala, R., Parthasarathi, , Patil, N., Verma, A., Waskar, M., et al. (2022). Interactions of Surfactants with the Bacterial Cell Wall and Inner Membrane: Revealing the Link between Aggregation and Antimicrobial Activity. Langmuir, 38(50), 15714-15728.
- Snook, E., Mitchell, T., Hinton, D. M., & Bacon, C. W. (2009). Isolation and characterization of Leu7-surfactin from the endophytic bacterium Bacillus mojavensis RRC 101, a biocontrol agent for Fusarium verticillioides. Journal of Agricultural and Food Chemistry, 57(10), 4287-4292.
- Stein, T., Heinzmann, , Solovieva, I., & Entian, K. D. (2003). Function of Lactococcus lactis nisin immunity genes nisI and nisFEG after coordinated expression in the surrogate host Bacillus subtilis. Journal of Biological Chemistry, 278(1), 89-94.
- Stein, T. (2005). Bacillus subtilis antibiotics: structures, syntheses and specific function Molecular Microbiology, 56(4), 845-857.
- Stein, T., Heinzmann, , Düsterhus, S., Borchert, S., & Entian, K. D. (2005). Expression and functional analysis of the subtilin immunity genes spaIFEG in the subtilin-sensitive host Bacillus subtilis MO1099. Journal of Bacteriology, 187(3), 822-828.
- Tambong, J. T., & Höfte, (2001). Phenazines are involved in biocontrol of Pythium myriotylum on cocoyam by Pseudomonas aeruginosa PNA1. European Journal of Plant Pathology, 107(5), 511-521.
- Tendulkar, , Saikumari, Y., Patel, V., Raghotama, S., Munshi, T., Balaram, P., & Chattoo, B. B. (2007). Isolation, purification and characterization of an antifungal molecule produced by Bacillus licheniformis BC98, and its effect on phytopathogen Magnaporthe grisea. Journal of Applied Microbiology, 103(6), 2331- 2339.
- Thomashow, S., & Weller, D. M. (1988). Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. Journal of Bacteriology, 170(8), 3499-3508.
- Tran, , Ficke, A., Asiimwe, T., Höfte, M., & Raaijmakers, J. M. (2007). Role of the cyclic lipopeptide massetolide A in biological control of Phytophthora infestans and in colonization of tomato plants by Pseudomonas fluorescens. New Phytologist, 175(4), 731-742.
- Tuomi, J., Niemelä, P., Haukioja, , Sirén, S., & Neuvonen, S. (1984). Nutrient stress: an explanation for plant anti-herbivore responses to defoliation. Oecologia, 61(2), 208-210.
- Villegas-Escobar, V., Ceballos, , Mira, J. J., Argel, L. E., Orduz Peralta, S., & Romero-Tabarez, M. (2013). Fengycin C produced by Bacillus subtilis EA-CB0015. Journal of Natural Products, 76(4), 503-509.
- Visca, P., Imperi, F., & Lamont, L. (2007). Pyoverdine siderophores: from biogenesis to biosignificance. Trends in Microbiology, 15(1), 22-30.
- Voisard, , Keel, C., Haas, D., & Dèfago, G. (1989). Cyanide production by Pseudomonas fluorescens helps suppress black root rot of tobacco under gnotobiotic conditions. The EMBO Journal, 8(2), 351-358.
- Weller, D. , Raaijmakers, J. M., Gardener, B. B. M., & Thomashow, L. S. (2002). Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annual Review of Phytopathology, 40(1), 309-348.
- Weller, D. (2007). Pseudomonas biocontrol agents of soilborne pathogens: looking back over 30 years. Phytopathology, 97(2), 250-256.
- Weller, D. , Mavrodi, D. V., van Pelt, J. A., Pieterse, C. M., van Loon, L. C., & Bakker, P. A. (2012). Induced systemic resistance in Arabidopsis thaliana against Pseudomonas syringae pv. tomato by 2,4-diacetylphloroglucinol-producing Pseudomonas fluorescens. Phytopathology, 102(4), 403-412.
- Wensing, , Braun, S. D., Büttner, P., Expert, D., Völksch, B., Ullrich, M. S., & Weingart, H. (2010). Impact of siderophore production by Pseudomonas syringae pv. syringae 22d/93 on epiphytic fitness and biocontrol activity against Pseudomonas syringae pv. glycinea 1a/96. Applied and Environmental Microbiology, 76(9), 2704-2711.
- Xu, Q., Pan, W., Zhang, R., Lu, Q., Xue, W., Wu, , et al. (2018). Inoculation with Bacillus subtilis and Azospirillum brasilense produces abscisic acid that reduces Irt1-mediated cadmium uptake of roots. Journal of Agricultural and Food Chemistry, 66(20), 5229-5236.
- Yánez-Mendizábal, V., Zeriouh, , Viñas, I., Torres, R., Usall, J., de Vicente, A., et al. (2012). Biological control of peach brown rot (Monilinia spp.) by Bacillus subtilis CPA-8 is based on production of fengycin-like lipopeptides. European Journal of Plant Pathology, 132(4), 609-619.
- Yoshida, , Hiradate, S., Tsukamoto, T., Hatakeda, K., & Shirata, A. (2001). Antimicrobial activity of culture filtrate of Bacillus amyloliquefaciens RC-2 isolated from mulberry leaves. Phytopathology, 91(2), 181-187.
- Zhao, X., Han, Y., Tan, X.-q., Wang, J., & Zhou, Z. J. (2014). Optimization of antifungal lipopeptide production from Bacillus sp. BH072 by response surface methodology. Journal of Microbiology, 52(4), 324-332.
- Zhao, Z., Wang, Q., Wang, K., Brian, K., Liu, C., & Gu, Y. (2010). Study of the antifungal activity of Bacillus vallismortis ZZ185 in vitro and identification of its antifungal componen Bioresource Technology, 101(1), 292-297.
- Živković, , Stojanović, S., Ivanović, Ž., Gavrilović, V., Popović, T., & Balaž, J. (2010). Screening of antagonistic activity of microorganisms against Colletotrichum acutatum and Colletotrichum gloeosporioides. Archives of Biological Sciences, 62(3), 611-623.
