1. Introduction
The human cytomegalovirus (CMV) belongs to the family Herpesviridae, a well-known group of pathogens that cause persistent infection within the host. A ubiquitous and metropolitan agent of infection, the CMV can reach a pervasiveness of nearly 100% in some areas of the world (Zuhair et al., 2019). It is mostly considered an innocuous pathogen in the healthy population. Conversely, in an immunocompromised setting, CMV can cause severe disease and lead to a fatal outcome.
The interaction between CMV and its host is a complex and manifold one, and the potential oncogenic capacity of the virus has been extensively debated. Cytomegalovirus has been associated with a vast spectrum of malignancies, including neoplasms of the brain, breast and the hematopoietic cell lineage (Stangherlin et al., 2016; Geisler et al., 2019; Francis et al., 2017; Wiemels et al., 2019). The faculties of the virus that speak in favour of oncogenicity, or at least oncomodulation (Herbein, 2018), seem to be ample. However, a number of studies imply an anti-tumour effect of CMV (Herbein, 2018; Kumar et al., 2016; Jurak & Brune 2006; Erkes et al., 2017). Finally, in recent years, mounting evidence supports a T-cell mediated host anti-cancer response targeting CMV molecules expressed on transformed cells (Cf. Discussion – The T-cell approach – from oncoprotection to a CMV-specific immunotherapy). The most studied of CNS neoplasia in this regard is the glioblastoma multiforme (GBM); gliomas indeed account for nigh on 80% of all primary brain neoplasia (Schwartzbaum et al., 2006). Notoriously, GBM is one of the most lethal tumours with a median survival of less than 15 months, even when the full therapeutic panoply of surgical intervention, radiation and chemotherapy is deployed against it (Imperato et al., 1990; Strupp et al., 2005). The first description of CMV in malignant glioma tissue was reported in 2002 by Cobbs and co-workers (Cobbs et al., 2002). Information about CMV presence in glioblastoma is still conflicting, and various opinions exist on the tumour/virus association.
Hitherto, we are yet to arrive at a categorical conclusion on the interplay between CMV and brain cancer, be it GBM or any other. Furthermore, most of the research done so far are single-centre studies employing in vitro or in vivo methodologies. Conflicting results also undermine the potential value of an immunotherapeutic approach. Additional molecular investigation into the CMV- and host- associated minutiae are certainly warranted. Finally, a global approach across a broad demographic swathe is lacking.
We inquired into the link between CMV perva- siveness and brain/CNS tumour incidences drawing from available population-wide data the world over. Therefore, the aim of this study was twofold: 1) to investigate the role CMV may play in CNS tumours from a global standpoint and 2) to summarize all hitherto published evidence from literature pertain- ing to CMV/CNS tumour/host interplay in a single comprehensive survey.
2. Materials and Methods
2.1 CMV and brain tumours – Global statistical analysis
For information on global cancer statistics for malignancies of the central nervous system, we accessed the World Health Organization Global Cancer Observatory (International Agency for Research on Cancer [IARC], 2020). All cancer histologies in the grouping “Cancer sites” were combined under an umbrella term “Brain, central nervous system” (B/CNS), and will be referred to as such from this point on, unless specified otherwise. The data was presented as age-adjusted annual incidence rates (per 105 persons) for both genders, encompassing the broadest age-range (0-85+ years) available. Incidences were observed for B/CNS malignancies monitored in 185 countries by the International Agency for Research on Cancer (IARC). As a control groups, we have chosen the incidences of Kaposi’s sarcoma (as the tumour is decidedly known to be caused by another pathogen, the Kaposi’s sarcoma-associated herpesvirus, or KSHV) and of all cancers combined. Cytomegalovirus pervasiveness was assessed based on the seroprevalence of the pathogen in 73 countries, as reported by Zuhair et al (2019). In order to inquire for potential association between CMV and CNS malignancies, the mentioned incidences and seroprevalences were subsequently compared by means of the Spearman’s rank correlation test. The same analysis was performed for KSHV and all cancers. All p-values <0.05 were considered significant.
2.2 CMV and brain tumours – Survey of published evidence
The PubMed® search engine was used in order to obtain all relevant literature. Studies listed in this manner were further interrogated for relevant publications, i.e., those that pertain to the underlying issue. Furthermore, works referenced within the acquired studies were additionally scoured for pertinent information and included herein. Original papers and reviews in the English language were utilized, along with papers with abstracts as the only available section of the work.
3. Results
The Spearman’s rank correlation test yielded a highly significant and inverse correlation between country- specific cancer incidences for B/CNV tumours and CMV seroprevalences (p=0.001, Spearman’s ρ=-0.541). The same significance and reverse association were attained when taking into account global incidence rates for all cancers combined (p=0.001, Spearman’s ρ=-0.732). Control dataset of KSHV incidences did not demonstrate statistical significance under the same circumstances (p=0.953, Spearman’s ρ=-0.007). It may be worthwhile noting that, while indeed no significance was observed, the correlation coefficient did likewise show an inverse association. Results of all three calculations are visually represented in Figure 1.
As for the survey of published evidence, the search utilizing the PubMed® engine yielded 270 results, with possible overlap due to repeating keywords. The results of the search are shown in Table 1.
4. Discussion
4.1 Cytomegalovirus – local infection, global protection?
The connection between CMV and tumours in general is still a matter of some debate. Although it certainly possesses an oncogenic panoply within its genome – and so far, frank induction of tumour formation cannot be unconditionally excluded it would seem more probable that CMV plays an oncomodulatory role in cancer pathogenesis. Conversely, evidence exists for an oncoprotective role of CMV in certain cases (Jankovic et al., 2022). Finally, in the last couple of decades, efforts to harness CMV for immunotherapeutic purposes have been underway – the notion of T-cell based cancer therapies that would home on CMV-derived epitopes has been meticulously explored.
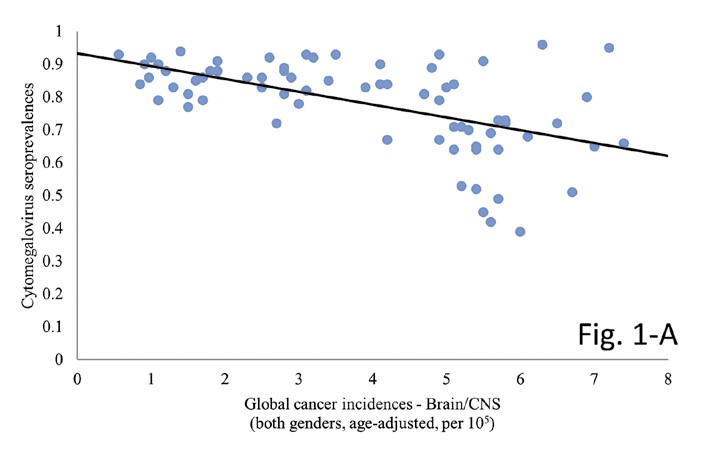
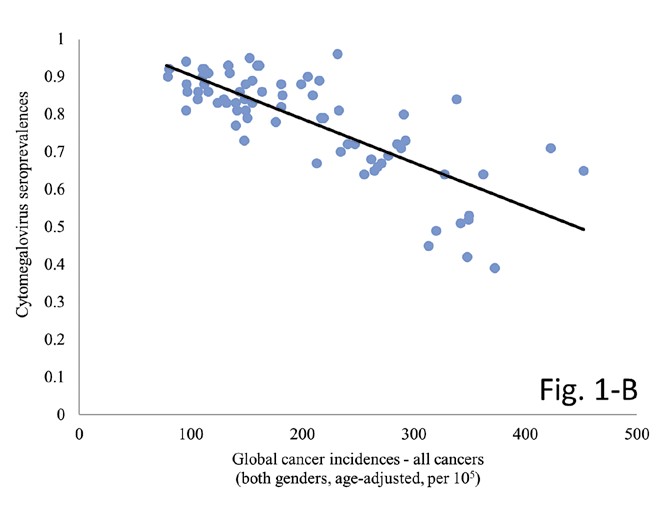

Figure 1. The graphs demonstrate the correlation between country-specific CMV prevalence and corresponding incidence rates for A) B/CNS tumours (p=0.001, Spearman’s ρ=-0.541), B) all cancers (p=0.001, Spearman’s ρ=-0.732) and C) Kaposi’s sarcoma (p=0.953, Spearman’s ρ=-0.007). Note the conspicuous lack of association for the control tumour incidences on graph C.
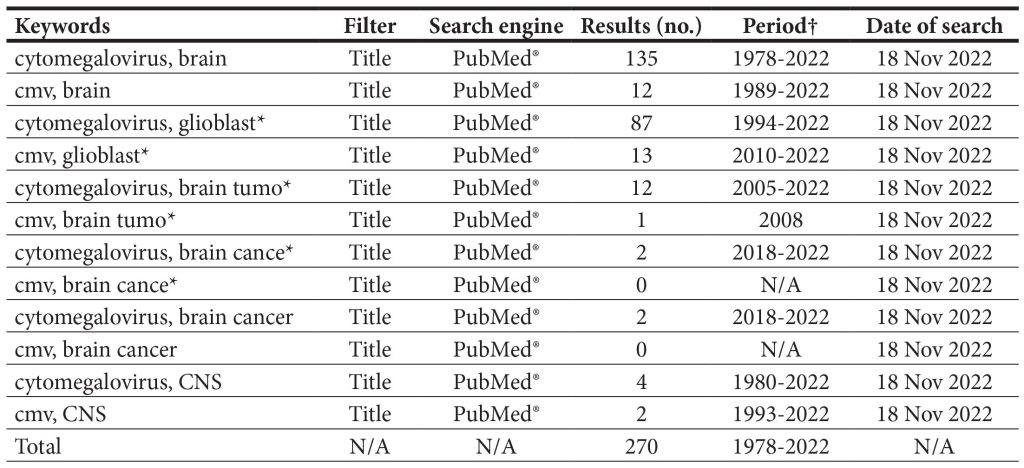
Table 1. The survey of literature as addressed by PubMed® search.
† The “Period” column indicates the time span in years during which all of the studies were published; N/A – not applicable.
As for the enigmatic affiliation between CMV and brain malignancies, the scientific limelight has so far predominantly focused on the CMV/GBM interplay; other tumours are mentioned only scarcely. The studies hitherto carried out are single-centre and in vitro studies. Additionally, a number of vaccine trials based on T-cells and dendritic cells have either been carried out, or are under way (Ahn et al., 2022).
This work aimed to summarize the existing knowledge on the interaction between the human CMV and brain tumours. Special attention will be given to glioblastomas, as the vast majority of research up to now concentrates on this entity.
Moreover, we are the first to report on a highly significant association between CMV and B/CNS tumours the world over which supports a potential oncoprotective capacity of the pathogen. So far, no study investigated the possible association that might become apparent only at across multiple country-wide landscapes. Our results speak in favour of an oncoprotective effect of CMV – as virus seroprevalences got higher, B/CNS incidences got significantly lower (p=0.001, Spearman’s ρ=-0.541). Interestingly, we have observed the same significant and inverse correlation across a wide spectrum (approximately 75%) of cancer histologies monitored by the World Health Organization (WHO) and IARC (author’s unpublished results, available upon reasonable request). The significance held when comparing incidences for all cancers combined (p=0.001, Spearman’s ρ=-0.732), as seen in Figure 1. The latter result would suggest a wide-ranging oncoprotective effect of CMV, not exclusive to brain tumours.
Unexpectedly, the lack of association between CMV and KSHV would also seem to speak in favour of a possible anti-tumour influence. Namely, a known mechanism by which CMV aids in eliminat- ing tumour cells is via T-cell mediated cancer cell de- struction. Kaposi’s sarcoma (KS) is rare and usually manifests in immunodeficiency, such as that caused by HIV. This tumour is the most frequent neoplasm in AIDS patients (Mercader et al., 2000), a known population which has a compromised T-cell immu- nity and diminished T-cell numbers. In this setting of a severely impaired cellular immunity, the reduced T-cell pool fails to control or eliminate KS – hence the lack in statistical reinforcement of the correlation.
4.2 Hidden, or in plain sight?
The ongoing discussion concerning the association between CMV and glioblastoma tumours is a polarized one. The very presence of CMV in glioblastoma is still a matter of some debate. The pervasiveness of viral infection has been widely deliberated, with the pathogen detected anywhere from almost 100% of samples, to the complete absence of CMV in neoplastic tissue. Hitherto, rate of CMV infection in glioblastoma is contentious and a categorical conclusion is so far lacking (Yang et al., 2022).
A comprehensive approach by Holdhoff and co-workers (Holdhoff et al., 2017) in which they uti- lized an array of highly sensitive detection methods demonstrated the utter lack of CMV in high grade glioblastoma tissues. Their conclusions are in concert with several other studies (Lau et al., 2005; Tang et al., 2013; Baumgarten et al., 2014; Yamashita et al., 2014; Tang et al., 2015; Strong et al., 2016; Lin et al., 2016; Garcia-Martinez et al., 2017; Taha et al., 2016), which also did not identify CMV in these malignancies. Re- cent and comprehensive investigation into the virus/ tumour interplay did not detect CMV across several CNS tumour histologies (medulloblastomas, pilo- cytic astrocytoma, glioblastomas and oligodendro- gliomas) (Zapatka et al., 2020). A study on 42 FFPE samples of tumour tissue from patients with GBM identified CMV in only 3 (7.1%) cases; furthermore, the patient group did not statistically differ from the control group that involved samples from subjects without brain disease (Ghaffari et al., 2021). Notably, EBV was herein significantly associated with GBM. Deep-coverage whole-genome sequencing was used in another study of tumours, where CMV DNA was not detected in 34 glioblastomas (Tang et al., 2015). Research aiming to detect CMV in high grade glio- mas and other paediatric brain tumours demonstrat- ed a clear lack of viral material in neoplastic cells of the examined cohort, be it by PCR or immunohisto- chemistry (Sardi et al., 2015).
The absence of CMV in GBM may stem from a number of reasons, not least of which is the meth- odology used in virus detection or the specific test used, as a number of methods have been employed for viral detection (Ahn et al., 2022). False positives due to antibody cross-reactivity and non-specific gli- al cell staining have been proposed (Korbecki et al., 2018). It may be argued as well that false negative re- sults were obtained in some of these laboratory stud- ies. However, in the study by Holdhoff et al. (2017) a number of rigorous methodologies were used, leav- ing the supposition of a laboratory error an unlike- ly explanation for the lack of CMV in glioblastoma tissues. The discrepancy in CMV detection from one laboratory setting to another could be due to vari- ances in control specimens, sample preparations and method sensitivity. Interestingly, disparate to other methods, all studies using next generation sequenc- ing (NGS) did not demonstrate the occurrence of CMV. One positive case was noted, however, but it could have resulted from contamination (Ahn et al., 2022). Achieving inter-laboratory uniformity in this regard could yield more precise results. Finally, the absence of CMV in glioblastoma may also be a con- sequence of the affinity of the virus towards a certain subtype of the malignancy, as there are four of those (Lehrer et al., 2011).
In contrast to research done in patients with glioblastoma that did not find CMV in tissue sam- ples, stands a variety of studies reporting on the de- tection of CMV in the majority of samples analysed (Cobbs et al., 2002; Rahbar et al., 2013; Stangherlin et al., 2016; Mitchell et al., 2008; Scheurer et al., 2008; Ranganathan et al., 2012; Libard et al., 2014; Sham- ran et al., 2015; Bhattacharjee et al., 2012). A recent systematic review of literature concluded that glio- blastoma tissue is highly pervaded with CMV and that optimal immunohistochemistry is mandated in order to detect presence of the pathogen (Pere- do-Harvey et al., 2021). In a study by Zavala-Vega et al. (2017), which aimed at detecting herpes simplex virus (HSV) and EBV in addition to CMV in brain tumours, the authors concluded that the viruses were found frequently in a highly seropositive popula- tion. It is interesting to note a work by Rahbar and colleagues (2013), which describes serology as an untrustworthy test for investigating the presence of prior or active CMV infection. Therein, the authors describe the presence of CMV in all tumours – how- ever, 29% of the patients were IgG negative. This in itself might imply that CMV pervasiveness is greater in patients with glioblastoma than expected, which may contradict our results attained from 73 coun- tries worldwide. The research was done on only 42 patients, however, and larger studies are warranted to substantiate the authors’ results of serology being an unreliable method. It has been suggested that, in or- der to remedy for the inconsistencies in CMV detec- tion among studies, a standardized protocol for viral detection likely using more than one method needs to be developed (Hochhalter et al., 2017).
4.3 Oncogenesis, oncomodulation or both?
So far, a division in opinions precludes a decided conclusion about the oncogenicity of CMV in B/CNS cancers. Literature provides leverage for both pro- oncogenic and anti-oncogenic capabilities of CMV. Nevertheless, a third option exists, where CMV can alter the course of an already established malignancy a property termed “oncomodulation”. A review by Solomon et al. states that there does not seem to be enough evidence for CMV as an agent of oncogenesis – rather, data would support an oncomodulatory role of the virus, excluding direct malignant transformation (Solomon et al., 2014). The infection with CMV may well be a double-edged sword – conferring protection early on in life, but harmful if it occurs later on in life. Namely, there is evidence for a temporal effect, where the pathogen may safeguard against glioblastoma multiforme in early childhood, while infection in later childhood and afterwards could pose as a causative factor (Lehrer, 2012).
Resent research employing single-cell RNA sequencing concluded that there was no decisive indication of full viral transcripts in analysed tumour tissue and cell lines (Johnson et al., 2017); low-abundance reads aligned across all tumours were also recognized. In a study of 116 Taiwanese subjects, researchers could not conclude an association between the virus and glioblastoma (Yang et al., 2017). The results from a study by Habibi and coworkers (2021) also could not support the role of CMV in non-glioblastoma multiforme infantile brain tumours. In a population of Japanese patients suffering from GBM, the authors did not find a connection between CMV and the cancer (Hashida et al., 2015), and a similar conclusion was reached elsewhere (Loit et al., 2019).
Interestingly, CMV seroprevalence rates are significantly lower in men than in women, even though glioblastoma is more common in men (Lehrer et al., 2015). This would suggest a potential oncoprotective capacity of CMV, which is in accordance with the results of our study. The use of oncolytic viruses to destroy neoplastic tissue is not a new concept (Hawkins & Croul, 2011). The CMV itself can cause direct cell death, along with “bystander” apoptosis; the brain pathology can even manifest via T-cell independent apoptosis of meningeal, glial and neuronal cells; this cell die-out was, however, demonstrated only in an immunodeficient mouse model (Reuter, 2005).
In another mouse model of glioma, the authors dispute the causal role of CMV as an agent of gliomagenesis; instead, they argue for an oncomodulatory effect of the virus, which leads to tumour-suppressor loss, thereby accelerating glioma formation/proliferation (Price et al., 2013). An oncomodulatory role of CMV has been proposed in brain malignancies including gliomas, medulloblastomas and neuroblastomas (El Baba & Herbein 2021). Oncomodulatory effects are most often explained as those that contribute to the increase of a tumour’s malignan- cy (Michaelis et al., 2009). We would be remiss not to mention, among others, the self-sufficiency in growth signals, insensitivity to anti-growth signals, evasion of apoptosis, limitless replicative potential, sustained angiogenesis, tissue invasion and metas- tasis, deregulation of cellular energetics, avoiding immune destruction, tumour-promoting inflam- mation, and genome instability and mutation – all specific onco-modulatory roles of CMV-infected glioma proposed by several investigations (El Baba & Herbein 2021; Herbein, 2018). The virus can also promote tumorigenesis and tumour invasion by cre- ating oncomodulatory proteins that interact with cancer cell pathways (Daei Sorkhabi et al., 2022). However, oncomodulation may not strictly imply an onco-promoting effect. Namely, in vitro research on glioblastoma multiforme cell lines by Dos Santos and colleagues concludes that the oncomodulatory po- tential of CMV also comprises a spectrum of hostile effects on tumour cell proliferation or survival, and is not restricted solely to cancer-promotion (Dos San- tos et al., 2018). Cytomegalovirus can also manifest an inhibiting effect on breast cancer cell migration by means of the virus glycoprotein B (Yang et al., 2018).
Korbecki et al. (2018) note in their work that there seems to be a poor correlation between CMV infection and glioblastoma multiforme epidemiolo- gy, as latent infection is present in over half the popu- lation, but the incidence of GBM is low (3/10000 per- sons/year). We offer a possible explanation for this phenomenon in our study; therein, changes in brain tumour incidences and CMV seroprevalence strong- ly and inversely associate the world over. Moreover, the authors’ have noted the high-CMV/low-GBM phenomenon, which speaks in favour of our conclu- sions that CMV might offers a degree of oncoprotec- tion. CMV is significantly less pervasive whites than in blacks or Hispanics (Mexican Americans), while glioblastoma incidence is higher in whites than in blacks or Hispanics (Lehrer et al., 2015). This is in line with our findings, as CMV is associated with a lower socioeconomic status (SES) – a social index unfortunately often found lower in blacks and His- panics alike. Supporting evidence hails from a Swed- ish study, which reports on increased odds of glioma in persons with a higher family income (adjustments were made age, sex and geographic region (Wigertz et al., 2010). Similar results are reported for those residing in areas with high SES, even after statistical adjustment for confounding factors – again, high- er rates for glioblastoma were demonstrated in this group (Chakrabarti et al., 2005). Having all else in mind, as this effect was observed around the world, we postulate that this protection is mediated via the T-cell CMV-aimed immune response at the very least.
Contrary to the potential oncoprotective capacity conceivably tied with CMV, the virus proteins could induce telomerase activity and angiogenesis; they could also control the cell cycle, inhibit apoptosis, activate the migration of cells and metastasis; finally, they could avoid immune-mediated obliteration, intensify genome instability, and endorse stemness via the obstruction of the differentiation of cells (Dziurzynski et al., 2012; Söderberg-Nauclér & Johnsen, 2015). Therefore, the pathogen’s occurrence in glioblastoma would uphold tumour progression.
An in vitro investigation done on U373MG cells (human glioblastoma cell line) aimed to study the ef- fect of the CMV IE1 protein on gene expression: the authors concluded that the IE1 gene product could modify the expression of genes that might be import- ant actors in the pathogenesis of glial tumours. No- tably, the expression of glial fibrillary acidic protein (GFAP), thrombospondin-1 (TSP-1), and p53 mRNA was decreased in cells expressing the aforementioned viral protein (Lee et al., 2005). An approach similar to our own was used by Lehrer et al. where the au- thors compared CMV seroprevalences and glioblas- toma incidence rates in the USA. Contrary to our findings, no consistent association between CMV and glioblastoma incidence was found (Lehrer et al., 2012). We postulate that this divergence may re- sult from different statistical approaches to available data. Moreover, while our results included all CNS tumours, the previous study was done solely consid- ering patients with glioblastoma. Finally, the global aspects in our study may simply not be apparent on a state-wide level.
Since the first description of CMV oncomodula- tion by Cinatl and colleagues nigh on 30 years ago (Cinatl et al., 1996a; Cinatl et al., 1996b), this ca- pacity of CMV has been extensively studied, along with the pros and cons for potential CMV tumori- genic effects. Holdhoff and colleagues (2017) have performed PCR on a portion of their patients, and the results were consistent with the rates of CMV se- ropositivity in the general population. This does not speak in favour of a potential oncoprotective effect of CMV in glioblastomas. It may be, however, that this anti-oncogenic property manifests in other types of tumours, or perhaps only in only a fraction of the patients with high grade glioma.
Interestingly, in a report from 2012 (Dziurzynski et al., 2012), it was summarized from the conclusions from The human cytomegalovirus (CMV) and gli- oma symposium that “existing evidence supports an oncomodulatory role for CMV in malignant gliomas, but future studies need to focus on determining the role of CMV as a glioma-initiating event”, as well as that “there is sufficient evidence to conclude that CMV sequences and viral gene expression exist in most, if not all, malignant gliomas…” – a stark contrast to a later research (Holdhoff et al., 2017). It seems there exists a close association between inflammation and tumour formation; viruses by themselves could in- duce an inflammatory environment, with emerging data pinning a pathogenic role on CMV in epithe- lial and neuronal malignancies (Söderberg-Nauclér & Johnsen, 2015). Another study speaks of CMV oncomodulation (and not necessarily oncogenesis) in which infection by the virus promoted epitheli- al-to-mesenchymal transition in glioblastoma cells and strengthened the invasiveness of glioma cells (Zhu et al., 2020).
An investigation into the molecular panoply of CMV has yielded conclusions that the virus induces the upregulation of transcripts of the MET oncogene (linked with a subset of glioblastoma multiforme patients) which may be a mechanism included in glioblastoma multiforme growth (Krenzlin et al., 2021). A significant increase in tumour growth was observed in mice infected with murine CMV, and the authors conclude that the virus potentiates glioblas- toma growth by increasing pericyte recruitment and angiogenesis. This is, in itself, an oncomodulating capacity of the murine CMV, and does not necessar- ily imply oncogenesis (Krenzlin et al., 2019). Matlaf and colleagues have suggested that the pp71 protein of CMV, previously demonstrated to promote cell cycle progression, can possibly be conducive to the aggressive phenotype of the glioblastoma multiforme (Matlaf et al., 2013). Finally, direct evidence for CMV oncogenicity – albeit in a mouse model – was demonstrated by Price and Chiocca (2015). To date, there is no decisive evidence to support this concept in humans.
4.4 The T-cell approach – from oncoprotection to CMV-specific immunotherapy
The last decade and a half saw a rise in interest for immunotherapy using T-cells that target GBM neo- plasia (Ahn et al., 2022). T-cells are known to elim- inate cancers existing in the immunologically priv- ileged environs of the central nervous system. Viral antigens that are present in malignant cells have been investigated as potential immunotherapeutic targets for quite some time, including cytotoxic T-lympho- cyte (CTL) or dendritic cell (DC)-based vaccines (Ahn et al., 2022). The idea of CMV presenting as a valid target for this therapeutic modality has also been discussed (Ahn et al., 2022). Cytomegalovirus antigens seem as a conspicuous bull’s eye for cellular immunotherapies in that they could present more potent tumour recognition sites than tumour-de- rived antigens themselves (Schuessler et al., 2014a). The assumption that CMV may act a target for can- cer treatment is not a novel one; with CMV being lo- calized within tumour cells, and healthy cells in close proximity remaining CMV-negative, it was proposed that the virus itself may prove as a fresh mark for therapeutic regimens against malignancy (Söder- berg-Nauclér & Johnsen, 2012).
As the notion of CMV epitopes as viable targets for the host immune system in tumour eradication seem to gain momentum, it is conceivable that the T-cells might “enforce” homeostasis more strongly in CMV infected individuals by eliminating GBM cells as they develop – an action we would call oncoprevention. This shielding effect conferred by “natural immunization” is supported by our findings of a global significant and inverse association between CMV seroprevalence and B/CNS tumour incidences.
The literature, again, extends conflicting opin- ions, although it would seem that the majority of studies speak in favour of an efficient T-cell mediated destruction of tumour tissue guided by CMV mol- ecules expressed with cancer cells; CMV is already widely accepted as a promising target for various immunotherapeutic approaches. The selective tro- pism of CMV for glial cells as an immunotherapeutic bull’s eye in patients suffering from glioblastoma was proposed some 15 years ago by Prins and colleagues (2008). The authors recognized the significance and simplicity of galvanizing a T-cell mediated anti-viral (and, by extension, anti-tumour) response against pathogenic, foreign epitopes instead of “self ” tumour antigens. It was also proposed that the improve- ment in the immunotherapeutic strategies against GBM be made by combining used endogenous with CMV-specific antigens expressed on tumour cells (Duinkerken et al., 2016). In a recent in-human trial of the therapeutic potential of cytomegalovirus-spe- cific adoptive cell therapy in participants with prima- ry GBM, in vitro–expanded autologous CMV-specif- ic T-cells were safely utilized as an adjuvant therapy (Smith et al., 2020). Of note, the authors concluded that overall survival may improve in these patients if the therapy was offered before recurrence. An- other earlier study suggested that a therapeutic reg- imen combining autologous CMV-specific T-cells and chemotherapy may offer clinical advantage in subjects afflicted by recurrent GBM (Schuessler et al., 2014b). Both research hint at a successful T-cell- vs-tumour phenomenon based on CMV molecules as targets – a possible mechanism that could be sub- stantiated by the results from our investigation. Im- munologic targeting of CMV infected cells is further supported by the notion that it can be achieved even with low levels of CMV gene expression (Prins et al., 2008); furthermore, destruction of infected cells by cytotoxic T-lymphocytes can happen with as few as 3 antigenic peptides on the cell’s surface (Purbhoo et al., 2004). In human models, it is elsewhere also indi- cated that antibody and T-cell reactivity to CMV (as well as Epstein-Barr virus) epitopes in those afflicted with glioblastoma or pancreatic cancer point to the antiviral immune response constituting an essential component of the in situ host protection against ma- lignancies (Meng et al., 2018).
The acuteness of a T-cell mediated tumoricid- al activity is underscored by Sampson and Mitchell (2011); the authors postulate that if CD133+ glioma stem cells do favourably express CMV antigens in vivo, therapies that mark CMV as a target may ex- ert a high degree of inhibition on malignant growth by exterminating this tumour-propagating group (Sampson & Mitchell, 2011). In a mouse mod- el, Brizic and colleagues (2018) demonstrated that adoptively transferred murine CMV-specific CD8+ T-cells provided protection to newborn mice against primary infection with this pathogen as well as re- duced brain pathology. These tissue-resident mem- ory T-cells controlled the latent murine CMV; with their depletion, the virus subsequently reactivated and led to heightened inflammation in the brain. This animal model would seem to advance the no- tion of T-cell importance in controlling CMV in the brain – and, by extension, perhaps brain tumours as well. Anti-CMV immunotherapy may owe its ef- ficiency to 1) the directing of the immune response towards virus protein-exhibiting cells that drive tu- mor growth, 2) activation of other immune cells that elicit further destruction of cells naïve for CMV, or 3) cross-priming after killing of cancer cells invaded by CMV (Rahman et al., 2018).
An additional argument may well be that early- stage immunotherapy-oriented trials with CMV as a target have yielded encouraging results (Lawler, 2015). It has been also suggested that new modalities using antiviral and immunotherapeutic approaches may have a role in combating the disease. Another study supports the rationale behind CMV-driven immunotherapy by demonstrating, among other conclusions, that CMV pp65-specific T-cells identify and destroy autologous glioblastoma multiforme cells (Nair et al., 2014a).
In a cohort of 49 newly diagnosed GBMs, another group of researchers have found the tell-tale viral proteins pp65 and pIE1 in roughly 50% of samples; the authors further speculate that cells positive for the virus can be recognized by T-cells specific for pp65/IE1 (Lucas et al., 2011). Recent research into pp65-specific cellular responses elicited in a regimen of dose-intensified temozolomide (TMZ) therapy accompanied by pp65-dendritic cells showed long- term progression-free survival (PFS) and overall survival (OS), lending further credence to studies targeting CMV in GBM (Batich et al., 2017). The median PFS and OS were 25.3 and 41.1 months, which is an encouraging time-span for a disease with a <15-month median survival regardless of surgical resection, high-dose radiation and TMZ. It was also shown that CMV-specific T-cells that recognized pp65- and IE1-expressing cells subsequently destroyed GBM cells infected with CMV. The authors of the study suggest that an infusion of these virus- aimed T-cell lines may be beneficial in patients with CMV-positive GBMs (Ghazi et al., 2012). The virus was also proposed as a therapeutic target in a cohort of 25 serially diagnosed paediatric glioblastoma multiforme patients, as the expression of pp65 and IE1-72 CMV antigens was present in the majority of cancer tissues (Wakefield et al., 2015). Although US28 of the CMV was described as contributing to the detrimental effects of GBM, the authors do suggest that the protein itself may prove a valid target in GMB treatment (Soroceanu et al., 2011).
An interesting find is described in a work by Crough et al. (2012); the researchers report on the status of CMV-specific CD8+ T-cells in GBM pa- tients already pervaded by the virus. The functional- ity of these cells in ex vivo analyses was found to be impaired; however, their faculties were re-established in vitro using CMV peptide epitopes and IL-2. This report on a rebound in T-cell functionality when ex- posed to CMV molecules also speaks in favour of the virus being a possibly potent cancer-destroying ad- junct to our own immune system, as well as to T-cell based therapies.
Studies testing dendritic cell therapies also seem to be promising; two papers report on more frequent occurrence of CMV-specific T-cells, along with better survival outcome, when using a CMV pp65- based dendritic cell vaccine (Mitchell et al., 2015; Reap et al., 2018). A promising nearly one third of patients which have undergone dendritic cell vaccine therapy in a report by Batich et al. have achieved exceptionally long survival (Batich et al., 2020). A recent work by Nair and colleagues endeavoured to stimulate T-cells from patients afflicted with GBM with autologous dendritic cells pulsed with viral RNA coding for the pp65, a known CMV protein. The researchers measured the function of the effector CMV pp65-specific T cells, and conclude that “CMV- specific T-cells can effectively target glioblastoma tumour cells for immunologic killing and support the rationale for the development of CMV-directed immunotherapy in patients with GBM” (Nair et al., 2014b).
There are studies, however, that present evidence against T-cell mediated cancer-suppression facilitated by CMV. Cancer associated environs might curb the usefulness of antiviral T-cells inside the tumour itself (Schuessler et al., 2014a). The tumour cellular composition prior to treatment may influence the effect of adoptive T-cell therapy (Walker et al., 2019). An experimental riposte to the supposition of T-cell mediated elimination of CMV-infected tumour cells comes from a study of Vδ2neg γδ T cells, which are important effectors against CMV. Herein, the authors show that the largest subset of these, the Vδ1+ γδ T cells, expanded/activated ex vivo, successfully recognize and eliminate established glioblastoma multiforme cell lines as well as and primary tumour- derived glioblastoma multiforme cells irrespective of CMV infection (Knight et al., 2013). Moreover, the authors note that the pathogen may well augment the resistance GBM cell lines to innate recognition. A study of 65 glioblastoma patients used highly- functional autologous polyclonal CMV pp65-specific T-cells (Weathers et al., 2020). The authors conclude that prior contact with CMV (i.e. seropositivity) does not vouch for the neoplasm to be susceptible to CMV-specific T-cells. This is hypothesised to be a consequence of heterogeneity in viral antigen expression; notably, the T-cell effector function was decreased. Furthermore, CMV itself has multiple ways to cause immune evasion. This is particularly apparent in glioblastoma stem cells (Schneider et al., 2016). It disrupts major histocompatibility complex (MHC) I: human leukocyte antigen (HLA)-A, HLA-B and HLA-C, the expression of which thwarts the recognition of the altered antigens on neoplastic cells (Trgovcich et al., 2006; Jackson et al., 2011; Noriega et al., 2012; Barel et al., 2003; Rölle & Olweus, 2009). Moreover, the pathogen can decrease the surface and increase the soluble HLA-G fraction (Onno et al., 2000; Pizzato et al., 2003) and produce the viral interleukin-10 (vIL-10) with an immunosuppressive property (Korbecki et al., 2018). These are only some of the ways CMV may aid in fending off the host’s immune arsenal. Moreover, the variable success of the T-cell based CMV-aimed immune response in patients with GBM may be attributed to an inconstant permissiveness of glioblastoma multiforme cells to CMV infection, as demonstrated in vitro (Dos Santos et al., 2018).
Finally, there are a number of unpublished trials that use the CMV-based approach, most of which did not, to our knowledge, made public their results (Ahn et al., 2022; Hochhalter et al., 2017). Overall, there now exists a substantial foundation for T-cell based vaccine approaches using CMV in cancer therapy. The results of our study are in line with these approaches and further support their rationale.
5. Conclusions
So far, the association between CMV and B/CNS tumours was broadly discussed. The literature is heavily reliant on CMV in glioblastoma, with other tumours mentioned only seldom. In this study, we have tried to recapitulate the sum of existing knowledge regarding this issue, and interpret our own results vis-à-vis the literature surveyed. Hitherto, there have been four hypotheses on the association between CMV and GBM, as elegantly summarized by Hochhalter and colleagues (2017):
- CMV is a bona fide causal agent of So far, except for a study in mice, no other evidence supports this claim;
- CMV may be oncomodulatory. As discussed above, oncomodulation is mostly equated with augmenting cancer progression, although there is evidence of oncomodulation that would benefit the patien Studies advocating for this property of CMV abound;
- CMV is present in GBM because of the im- munosuppressive tumour microenvironment;
- The detection of CMV in GBM is merely a laboratory artefact, a consequence of various methodological glitch
However, mounting evidence would support an anti-cancer role of CMV in general. As regards GBM, this is reflected in the success of CMV-based T-cell therapies, our most recent results that hint at CMV- related oncoprotection in B/CNS tumours, as well as other research mentioned above (Cf. Discussion). Having all this in mind, we find it prudent to put forward a fifth premise:
- CMV may offer a degree of oncoprotection and/or oncoprevention agent against
Up until now there hasn’t been any conclusive correlation between CMV seropositivity and glioblastoma incidence. Furthermore, the literature is still conflicting on the presence of CMV in glioblastoma cells, which may limit the potential usefulness of CMV-based therapeutic approaches. Our study is the first to hint at a phenomenon of CMV protection against B/CNS tumours on a global scale, and corroborates all similar experimental results reported elsewhere. Correlation does not have to infer causation, however, and these results should be interpreted with caution – much still remains obscure in the domain of CMV oncogenicity.
A complete understanding of the interplay be- tween CMV and glioblastoma – and brain tumours in general – still eludes us. The novel evidence emer- gent from a global scale that CMV potentially may be oncoprotective speak in favour of the importance of T-cell mediated immunotherapies. More compre- hensive molecular studies investigating host-patho- gen interactions, as well as large multicentre studies and broad-swathed epidemiological inquiries are needed to corroborate our results and hopefully fi- nally untie a Gordian knot of a longstanding debate.
Supplementary Materials: Not applicable. Funding: I would like to acknowledge the research funding by the MPNTR grant of the National Ministry of Education, Science and Technological Development, project No. 1750-73. The National Ministry as the funding source had no role in study design, data collection and interpretation, or the decision to submit this work for publication.
Informed Consent Statement: Not applicable.
Data Availability Statement: All data is available from the author upon reasonable request.
Acknowledgments: My deepest gratitude goes out to Professors Tanja Jovanovic, Aleksandra Knezevic and Assistant Professor Ina Gajic for their invaluable counsel and support in all scientific matters. I would like to acknowledge the research funding by the MPNTR grant of the National Ministry of Education, Science and Technological Development, project No. 1750-73.
Conflicts of Interest: The author declares no conflicts of interest.
References
- Ahn, J., Shin, , Kim, Y. S., Park, J. S., Jeun, S. S., & Ahn, S. (2022). Cytomegalovirus-Specific Immunotherapy for Glioblastoma Treatments. Brain tumor research and treatment, 10(3), 135–143.
- Barel, T., Ressing, M., Pizzato, N., van Leeuwen, D., Le Bouteiller, P., Lenfant, F., & Wiertz, E. J. (2003). Human cytomegalovirus-encoded US2 differentially affects surface expression of MHC class I locus products and targets membrane-bound, but not soluble HLA-G1 for degradation. Journal of immunology (Baltimore, Md. : 1950), 171(12), 6757–6765.
- Batich, A., Mitchell, D. A., Healy, P., Herndon, J. E., 2nd, & Sampson, J. H. (2020). Once, Twice, Three Times a Finding: Reproducibility of Dendritic Cell Vaccine Trials Targeting Cytomegalovirus in Glioblastoma. Clinical cancer research : an official journal of the American Association for Cancer Research, 26(20), 5297–5303.
- Batich, K. A., Reap, E. A., Archer, G. E., Sanchez- Perez, , Nair, S. K., Schmittling, R. J., Norberg, P., Xie, W., Herndon, J. E., 2nd, Healy, P., McLendon, R. E., Friedman, A. H., Friedman, H. S., Bigner, D., Vlahovic, G., Mitchell, D. A., & Sampson, J. H. (2017). Long-term Survival in Glioblastoma with Cytomegalovirus pp65- Targeted Vaccination. Clinical cancer research : an official journal of the American Association for Cancer Research, 23(8), 1898–1909.
- Baumgarten, P., Michaelis, , Rothweiler, F., Starzetz, T., Rabenau, H. F., Berger, A., Jennewein, L., Braczynski, A. K., Franz, K., Seifert, V., Steinbach, J. P., Allwinn, R., Mittelbronn, M., & Cinatl, J., Jr (2014). Human cytomegalovirus infection in tumor cells of the nervous system is not detectable with standardized pathologico-virological diagnostics. Neuro-oncology, 16(11), 1469–1477.
- Bhattacharjee, B., Renzette, , & Kowalik, T. F. (2012). Genetic analysis of cytomegalovirus in malignant gliomas. Journal of virology, 86(12), 6815–6824.
- Brizić, , Šušak, B., Arapović, M., Huszthy, P. C., Hiršl, L., Kveštak, D., Juranić Lisnić, V., Golemac, M., Pernjak Pugel, E., Tomac, J., Oxenius, A., Britt, W. J., Arapović, J., Krmpotić, A., & Jonjić, S. (2018). Brain- resident memory CD8+ T cells induced by congenital CMV infection prevent brain pathology and virus reactivation. European journal of immunology, 48(6), 950–964.
- Chakrabarti, , Cockburn, M., Cozen, W., Wang, Y. P., & Preston-Martin, S. (2005). A population-based description of glioblastoma multiforme in Los Angeles County, 1974-1999. Cancer, 104(12), 2798–2806.
- Cinatl, J., Jr, Cinatl, J., Vogel, J. U., Rabenau, , Kornhuber, B., & Doerr, H. W. (1996). Modulatory effects of human cytomegalovirus infection on malignant properties of cancer cells. Intervirology, 39(4), 259–269.
- Cinatl, J., Jr, Vogel, J. U., Cinatl, J., Weber, B., Rabenau, , Novak, M., Kornhuber, B., & Doerr, H. W. (1996). Long-term productive human cytomegalovirus infection of a human neuroblastoma cell line. International journal of cancer, 65(1), 90–96.
- Cobbs, S., Harkins, L., Samanta, M., Gillespie, G. Y., Bharara, S., King, P. H., Nabors, L. B., Cobbs, C. G., & Britt, W. J. (2002). Human cytomegalovirus infection and expression in human malignant glioma. Cancer research, 62(12), 3347–3350.
- Crough, T., Beagley, , Smith, C., Jones, L., Walker, D. G., & Khanna, R. (2012). Ex vivo functional analysis, expansion and adoptive transfer of cytomegalovirus- specific T-cells in patients with glioblastoma multiforme. Immunology and cell biology, 90(9), 872– 880.
- Daei Sorkhabi, , Sarkesh, A., Saeedi, H., Marofi, F., Ghaebi, M., Silvestris, N., Baradaran, B., & Brunetti, O. (2022). The Basis and Advances in Clinical Application of Cytomegalovirus-Specific Cytotoxic T Cell Immunotherapy for Glioblastoma Multiforme. Frontiers in oncology, 12, 818447.
- Dos Santos, J., Ferreira Castro, F. L., de Aguiar, R. B., Menezes, I. G., Santos, A. C., Paulus, C., Nevels, M., & Carlan da Silva, M. C. (2018). Impact of human cytomegalovirus on glioblastoma cell viability and chemotherapy treatment. The Journal of general virology, 99(9), 1274–1285.
- Duinkerken, S., van Kooyk, Y., & Garcia-Vallejo, J. J. (2016). Human cytomegalovirus-based immuno- therapy to treat glioblastoma: Into the future. Oncoimmunology, 5(9),
- Dziurzynski, , Chang, S. M., Heimberger, A. B., Kalejta, R. F., McGregor Dallas, S. R., Smit, M., Soroceanu, L., Cobbs, C. S., & HCMV and Gliomas Symposium (2012). Consensus on the role of human cytomegalovirus in glioblastoma. Neuro-oncology, 14(3), 246–255.
- El Baba, R., & Herbein, (2021). Immune Landscape of CMV Infection in Cancer Patients: From “Canonical” Diseases Toward Virus-Elicited Oncomodulation. Frontiers in immunology, 12, 730765.
- Erkes, D. , Wilski, N. A., & Snyder, C. M. (2017). Intratumoral infection by CMV may change the tumor environment by directly interacting with tumor- associated macrophages to promote cancer immunity. Human vaccines & immunotherapeutics, 13(8), 1778–1785.
- Francis, S., Wallace, A. D., Wendt, G. A., Li, L., Liu, ., Riley, L. W., Kogan, S., Walsh, K. M., de Smith, A. J., Dahl, G. V., Ma, X., Delwart, E., Metayer, C., & Wiemels, J. L. (2017). In utero cytomegalovirus infection and development of childhood acute lymphoblastic leukemia. Blood, 129(12), 1680–1684.
- Garcia-Martinez, , Alenda, C., Irles, E., Ochoa, E., Quintanar, T., Rodriguez-Lescure, A., Soto, J. L., & Barbera, V. M. (2017). Lack of cytomegalovirus detection in human glioma. Virology journal, 14(1), 216.
- Geisler, J., Touma, J., Rahbar, , Söderberg-Nauclér, C., & Vetvik, K. (2019). A Review of the Potential Role of Human Cytomegalovirus (HCMV) Infections in Breast Cancer Carcinogenesis and Abnormal Immunity. Cancers, 11(12), 1842.
- Ghaffari, , Tavakoli, A., Faranoush, M., Naderi, A., Kiani, S. J., Sadeghipour, A., Javanmard, D., Farahmand, M., Ghorbani, S., Sedaghati, F., & Monavari, S. H. (2021). Molecular Investigation of Human Cytomegalovirus and Epstein-Barr virus in Glioblastoma Brain Tumor: A Case-Control Study in Iran. Iranian biomedical journal, 25(6), 426–433.
- Ghazi, , Ashoori, A., Hanley, P. J., Brawley, V. S., Shaffer, D. R., Kew, Y., Powell, S. Z., Grossman, R., Grada, Z., Scheurer, M. E., Hegde, M., Leen, A. M., Bollard, C. M., Rooney, C. M., Heslop, H. E., Gottschalk, S., & Ahmed, N. (2012). Generation of polyclonal CMV-specific T cells for the adoptive immunotherapy of glioblastoma. Journal of immunotherapy (Hagerstown, Md. : 1997), 35(2), 159–168.
- Habibi, , Hajizadeh, M., Nozarian, Z., Safavi, M., Monajemzadeh, M., Meybodi, K. T., Nejat, F., & Vasei, M. (2021). Cytomegalovirus DNA in non-glioblastoma multiforme brain tumors of infants. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery, 37(5), 1581–1586.
- Hashida, Y., Taniguchi, , Yawata, T., Hosokawa, S., Murakami, M., Hiroi, M., Ueba, T., & Daibata, M. (2015). Prevalence of human cytomegalovirus, polyomaviruses, and oncogenic viruses in glioblastoma among Japanese subjects. Infectious agents and cancer, 10, 3.
- Hawkins, , & Croul, S. (2011). Viruses and human brain tumors: cytomegalovirus enters the fray. The Journal of clinical investigation, 121(10), 3831–3833.
- Herbein (2018). The Human Cytomegalovirus, from Oncomodulation to Oncogenesis. Viruses, 10(8), 408.
- Hochhalter B., Carr C., O’Neill B. E., Ware M. L., & Strong M. J. (2017). The association between human cytomegalovirus and glioblastomas: a review. Neuroimmunol Neuroinflammation 4:96-108.
- Holdhoff, M., Guner, G., Rodriguez, F. J., Hicks, J. L., Zheng, Q., Forman, M. S., Ye, X., Grossman, S. A., Meeker, A. K., Heaphy, C. M., Eberhart, C. ., De Marzo, A. M., & Arav-Boger, R. (2017). Absence of Cytomegalovirus in Glioblastoma and Other High-grade Gliomas by Real-time PCR, Immunohistochemistry, and In Situ Hybridizatio Clinical cancer research : an official journal of the American Association for Cancer Research, 23(12), 3150–3157.
- Imperato, J. P., Paleologos, A., & Vick, N. A. (1990). Effects of treatment on long-term survivors with malignant astrocytomas. Annals of neurology, 28(6), 818–822.
- International Agency for Research on Cancer (2020). Global Cancer Observatory. Retrieved from https://gco.iarfr/.
- Jackson, E., Mason, G. M., & Wills, M. R. (2011). Human cytomegalovirus immunity and immune evasion. Virus research, 157(2), 151–160.
- Janković, , Knežević, A., Todorović, M., Đunić, I., Mihaljević, B., Soldatović, I., Protić, J., Miković, N., Stoiljković, V., & Jovanović, T. (2022). Cytomegalovirus infection may be oncoprotective against neoplasms of B-lymphocyte lineage: single-institution experience and survey of global evidence. Virology journal, 19(1), 155.
- Johnson, T. , Abrams, Z. B., Mo, X., Zhang, Y., & Huang, K. (2017). Lack of human cytomegalovirus expression in single cells from glioblastoma tumors and cell lines. Journal of neurovirology, 23(5), 671–678.
- Jurak, , & Brune, W. (2006). Induction of apoptosis limits cytomegalovirus cross-species infection. The EMBO journal, 25(11), 2634–2642.
- Knight, , Arnouk, H., Britt, W., Gillespie, G. Y., Cloud, G. A., Harkins, L., Su, Y., Lowdell, M. W., & Lamb, L. S. (2013). CMV-independent lysis of glioblastoma by ex vivo expanded/activated Vδ1+ γδ T cells. PloS one, 8(8), e68729.
- Korbecki, J., Gutowska, I., Kojder, I., Jeżewski, D., Goschorska, M., Łukomska, A., Lubkowska, , Chlubek, D., & Baranowska-Bosiacka, I. (2018). New extracellular factors in glioblastoma multiforme development: neurotensin, growth differentiation factor-15, sphingosine-1-phosphate and cytomegalovirus infection. Oncotarget, 9(6), 7219–7270.
- Krenzlin, , Behera, P., Lorenz, V., Passaro, C., Zdioruk, M., Nowicki, M. O., Grauwet, K., Zhang, H., Skubal, M., Ito, H., Zane, R., Gutknecht, M., Griessl, M. B., Ricklefs, F., Ding, L., Peled, S., Rooj, A., James, C. D., Cobbs, C. S., Cook, C. H., … Lawler, S. E. (2019). Cytomegalovirus promotes murine glioblastoma growth via pericyte recruitment and angiogenesis. The Journal of clinical investigation, 129(4), 1671–1683.
- Krenzlin, , Zdioruk, M., Nowicki, M. O., Finkelberg, T., Keric, N., Lemmermann, N., Skubal, M., Chiocca, E. A., Cook, C. H., & Lawler, S. E. (2021). Cytomegalovirus infection of glioblastoma cells leads to NF-κB dependent upregulation of the c-MET oncogenic tyrosine kinase. Cancer letters, 513, 26–35.
- Kumar, , Coquard, L., Pasquereau, S., Russo, L., Valmary-Degano, S., Borg, C., Pothier, P., & Herbein, G. (2016). Tumor control by human cytomegalovirus in a murine model of hepatocellular carcinoma. Molecular therapy oncolytics, 3, 16012.
- Lau, K., Chen, Y. Y., Chen, W. G., Diamond, D. J., Mamelak, A. N., Zaia, J. A., & Weiss, L. M. (2005). Lack of association of cytomegalovirus with human brain tumors. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc, 18(6), 838–843.
- Lawler E. (2015). Cytomegalovirus and glioblastoma; controversies and opportunities. Journal of neuro- oncology, 123(3), 465–471.
- Lee, , Jeon, K., Kim, J. M., Kim, V. N., Choi, D. H., Kim, S. U., & Kim, S. (2005). Downregulation of GFAP, TSP- 1, and p53 in human glioblastoma cell line, U373MG, by IE1 protein from human cytomegalovirus. Glia, 51(1), 1–12.
- Lehrer S. (2012). Cytomegalovirus infection in early childhood may be protective against glioblastoma multiforme, while later infection is a risk factor. Medical hypotheses, 78(5), 657–658.
- Lehrer, , Green, S., Ramanathan, L., Rosenzweig, K., & Labombardi, V. (2012). No consistent relationship of glioblastoma incidence and cytomegalovirus seropositivity in whites, blacks, and Hispanics. Anticancer research, 32(3), 1113–1115.
- Lehrer, , Green, S., Rosenzweig, K. E., & Rendo, A. (2015). No circulating human cytomegalovirus in 14 cases of glioblastoma. Neuro-oncology, 17(2), 320.
- Lehrer, , Labombardi, V., Green, S., Pessin-Minsley, M. S., Germano, I. M., & Rosenzweig, K. E. (2011). No circulating cytomegalovirus in five patients with glioblastoma multiforme. Anticancer research, 31(3), 959–960.
- Libard, , Popova, S. N., Amini, R. M., Kärjä, V., Pietiläinen, T., Hämäläinen, K. M., Sundström, C., Hesselager, G., Bergqvist, M., Ekman, S., Zetterling, M., Smits, A., Nilsson, P., Pfeifer, S., de Ståhl, T. D., Enblad, G., Ponten, F., & Alafuzoff, I. (2014). Human cytomegalovirus tegument protein pp65 is detected in all intra- and extra-axial brain tumours independent of the tumour type or grade. PloS one, 9(9), e108861.
- Lin, T., Leibovitch, E. C., Almira-Suarez, M. I., & Jacobson, S. (2016). Human herpesvirus multiplex ddPCR detection in brain tissue from low- and high- grade astrocytoma cases and controls. Infectious agents and cancer, 11, 32.
- Loit, P., Adle-Biassette, H., Bouazza, S., Mazeron, M. C., Manivet, P., Lehmann-Che, J., Teissier, N., Mandonnet, E., & Molina, J. M. (2019). Multimodal techniques failed to detect cytomegalovirus in human glioblastoma samples. Journal of neurovirology, 25(1), 50–56.
- Lucas, G., Bao, L., Bruggeman, R., Dunham, K., & Specht, C. (2011). The detection of CMV pp65 and IE1 in glioblastoma multiforme. Journal of neuro- oncology, 103(2), 231–238.
- Matlaf, A., Harkins, L. E., Bezrookove, V., Cobbs, C. S., & Soroceanu, L. (2013). Cytomegalovirus pp71 protein is expressed in human glioblastoma and promotes pro-angiogenic signaling by activation of stem cell factor. PloS one, 8(7), e68176.
- Meng, Q., Valentini, D., Rao, M., Dodoo, E., & Maeurer, (2018). CMV and EBV targets recognized by tumor-infiltrating B lymphocytes in pancreatic cancer and brain tumors. Scientific reports, 8(1), 17079.
- Mercader, , Taddeo, B., Panella, J. R., Chandran, B., Nickoloff, B. J., & Foreman, K. E. (2000). Induction of HHV-8 lytic cycle replication by inflammatory cytokines produced by HIV-1-infected T cells. The American journal of pathology, 156(6), 1961–1971.
- Michaelis, , Doerr, H. W., & Cinatl, J. (2009). The story of human cytomegalovirus and cancer: increasing evidence and open questions. Neoplasia (New York, N.Y.), 11(1), 1–9.
- Mitchell, D. , Batich, K. A., Gunn, M. D., Huang, M. N., Sanchez-Perez, L., Nair, S. K., Congdon, K. L., Reap, E. A., Archer, G. E., Desjardins, A., Friedman, A. H., Friedman, H. S., Herndon, J. E., 2nd, Coan, A., McLendon, R. E., Reardon, D. A., Vredenburgh, J. J., Bigner, D. D., & Sampson, J. H. (2015). Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature, 519(7543), 366–369.
- Mitchell, D. , Xie, W., Schmittling, R., Learn, C., Friedman, A., McLendon, R. E., & Sampson, J. H. (2008). Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro-oncology, 10(1), 10–18.
- Nair, K., De Leon, G., Boczkowski, D., Schmittling, R., Xie, W., Staats, J., Liu, R., Johnson, L. A., Weinhold, K., Archer, G. E., Sampson, J. H., & Mitchell, D. A. (2014). Recognition and killing of autologous, primary glioblastoma tumor cells by human cytomegalovirus pp65-specific cytotoxic T cells. Clinical cancer research: an official journal of the American Association for Cancer Research, 20(10), 2684–2694.
- Nair, K., Sampson, J. H., & Mitchell, D. A. (2014). Immunological targeting of cytomegalovirus for glioblastoma therapy. Oncoimmunology, 3, e29289.
- Noriega, V., Redmann, V., Gardner, T., & Tortorella, D. (2012). Diverse immune evasion strategies by human cytomegaloviru Immunologic research, 54(1-3), 140–151.
- Onno, , Pangault, C., Le Friec, G., Guilloux, V., André, P., & Fauchet, R. (2000). Modulation of HLA-G antigens expression by human cytomegalovirus: specific induction in activated macrophages harboring human cytomegalovirus infection. Journal of immunology (Baltimore, Md. : 1950), 164(12), 6426–6434.
- Peredo-Harvey, I., Rahbar, A., & Söderberg-Nauclér, C. (2021). Presence of the Human Cytomegalovirus in Glioblastomas-A Systematic Review. Cancers, 13(20), 5051.
- Pizzato, , Garmy-Susini, B., Le Bouteiller, P., & Lenfant, F. (2003). Down-regulation of HLA-G1 cell surface expression in human cytomegalovirus infected cells. American journal of reproductive immunology (New York, N.Y. : 1989), 50(4), 328–333.
- Price, R. , & Chiocca, E. A. (2015). Modeling cytomegalovirus infection in mouse tumor models. Frontiers in oncology, 5, 61.
- Price, R. , Song, J., Bingmer, K., Kim, T. H., Yi, J. Y., Nowicki, M. O., Mo, X., Hollon, T., Murnan, E., Alvarez-Breckenridge, C., Fernandez, S., Kaur, B., Rivera, A., Oglesbee, M., Cook, C., Chiocca, E. A., & Kwon, C. H. (2013). Cytomegalovirus contributes to glioblastoma in the context of tumor suppressor mutations. Cancer research, 73(11), 3441–3450.
- Prins, R. , Cloughesy, T. F., & Liau, L. M. (2008). Cytomegalovirus immunity after vaccination with autologous glioblastoma lysate. The New England journal of medicine, 359(5), 539–541.
- Purbhoo, A., Irvine, D. J., Huppa, J. B., & Davis, M. M. (2004). T cell killing does not require the formation of a stable mature immunological synapse. Nature immunology, 5(5), 524–530.
- Rahbar, , Orrego, A., Peredo, I., Dzabic, M., Wolmer- Solberg, N., Strååt, K., Stragliotto, G., & Söderberg- Nauclér, C. (2013). Human cytomegalovirus infection levels in glioblastoma multiforme are of prognostic value for survival. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology, 57(1), 36–42.
- Rahman, , Dastmalchi, F., Karachi, A., & Mitchell, D. (2018). The role of CMV in glioblastoma and implications for immunotherapeutic strategies. Oncoimmunology, 8(1), e1514921.
- Ranganathan, P., Clark, P. , Kuo, J. S., Salamat, M. S., & Kalejta, R. F. (2012). Significant association of multiple human cytomegalovirus genomic Loci with glioblastoma multiforme samples. Journal of virology, 86(2), 854–864.
- Reap, A., Suryadevara, C. M., Batich, K. A., Sanchez- Perez, L., Archer, G. E., Schmittling, R. J., Norberg, P. K., Herndon, J. E., 2nd, Healy, P., Congdon, K. L., Gedeon, P. C., Campbell, O. C., Swartz, A. M., Riccione, K. A., Yi, J. S., Hossain-Ibrahim, M. K., Saraswathula, A., Nair, S. K., Dunn-Pirio, A. M., Broome, T. M., … Sampson, J. H. (2018). Dendritic Cells Enhance Polyfunctionality of Adoptively Transferred T Cells That Target Cytomegalovirus in Glioblastoma. Cancer research, 78(1), 256–264.
- Reuter J. D. (2005). Cytomegalovirus induces T-cell independent apoptosis in brain during immunodeficiency. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology, 32(3), 218–223.
- Rölle, A., & Olweus, J. (2009). Dendritic cells in cytomegalovirus infection: viral evasion and host countermeasur APMIS : acta pathologica, microbiologica, et immunologica Scandinavica, 117(5- 6), 413–426.
- Sampson, J. , & Mitchell, D. A. (2011). Is cytomegalovirus a therapeutic target in glioblastoma?. Clinical cancer research : an official journal of the American Association for Cancer Research, 17(14), 4619–4621.
- Sardi, , Lucchesi, M., Becciani, S., Facchini, L., Guidi, M., Buccoliero, A. M., Moriondo, M., Baroni, G., Stival, A., Farina, S., Genitori, L., & de Martino, M. (2015). Absence of human cytomegalovirus infection in childhood brain tumors. American journal of cancer research, 5(8), 2476–2483.
- Scheurer, E., Bondy, M. L., Aldape, K. D., Albrecht, T., & El-Zein, R. (2008). Detection of human cytomegalovirus in different histological types of gliomas. Acta neuropathologica, 116(1), 79–86.
- Schneider, , Ströbele, S., Nonnenmacher, L., Siegelin, M. D., Tepper, M., Stroh, S., Hasslacher, S., Enzenmüller, S., Strauss, G., Baumann, B., Karpel- Massler, G., Westhoff, M. A., Debatin, K. M., & Halatsch, M. E. (2016). A paired comparison between glioblastoma “stem cells” and differentiated cells. International journal of cancer, 138(7), 1709–1718.
- Schuessler, , Smith, C., Beagley, L., Boyle, G. M., Rehan, S., Matthews, K., Jones, L., Crough, T., Dasari, V., Klein, K., Smalley, A., Alexander, H., Walker, D. G., & Khanna, R. (2014). Autologous T-cell therapy for cytomegalovirus as a consolidative treatment for recurrent glioblastoma. Cancer research, 74(13), 3466–3476.
- Schuessler, A., Walker, D. G., & Khanna, R. (2014). Cytomegalovirus as a novel target for immunotherapy of glioblastoma multiforme. Frontiers in oncology, 4, 275.
- Schwartzbaum, J. , Fisher, J. L., Aldape, K. D., & Wrensch, M. (2006). Epidemiology and molecular pathology of glioma. Nature clinical practice. Neurology, 2(9), 494–516.
- Shamran, H. A., Kadhim, H. S., Hussain, A. R., Kareem, , Taub, D. D., Price, R. L., Nagarkatti, M., Nagarkatti, P. S., & Singh, U. P. (2015). Detection of human cytomegalovirus in different histopathological types of glioma in Iraqi patients. BioMed research international, 2015, 642652.
- Smith, , Lineburg, K. E., Martins, J. P., Ambalathingal, G. R., Neller, M. A., Morrison, B., Matthews, K. K., Rehan, S., Crooks, P., Panikkar, A., Beagley, L., Le Texier, L., Srihari, S., Walker, D., & Khanna, R. (2020). Autologous CMV-specific T cells are a safe adjuvant immunotherapy for primary glioblastoma multiforme. The Journal of clinical investigation, 130(11), 6041–6053.
- Söderberg-Nauclér, C., & Johnsen, J. I. (2012). Cytomegalovirus infection in brain tumors: A potential new target for therapy?. Oncoimmunology, 1(5), 739–740.
- Söderberg-Nauclér, C., & Johnsen, J. I. (2015). Cytomegalovirus in human brain tumors: Role in pathogenesis and potential treatment option World journal of experimental medicine, 5(1), 1–10.
- Solomon, H., Ramkissoon, S. H., Milner, D. A., Jr, & Folkerth, R. D. (2014). Cytomegalovirus and glioblastoma: a review of evidence for their association and indications for testing and treatment. Journal of neuropathology and experimental neurology, 73(11), 994–998.
- Soroceanu, , Matlaf, L., Bezrookove, V., Harkins, L., Martinez, R., Greene, M., Soteropoulos, P., & Cobbs, C. S. (2011). Human cytomegalovirus US28 found in glioblastoma promotes an invasive and angiogenic phenotype. Cancer research, 71(21), 6643–6653.
- Stangherlin, M., Castro, F. L., Medeiros, R. S., Guerra, J. M., Kimura, L. M., Shirata, N. K., Nonogaki, S., Dos Santos, C. J., & Carlan Silva, M. C. (2016). Human Cytomegalovirus DNA Quantification and Gene Expression in Gliomas of Different Grades. PloS one, 11(7), e0159604.
- Strong, J., Blanchard, E., 4th, Lin, Z., Morris, C. A., Baddoo, M., Taylor, C. M., Ware, M. L., & Flemington, E. K. (2016). A comprehensive next generation sequencing-based virome assessment in brain tissue suggests no major virus – tumor association. Acta neuropathologica communications, 4(1), 71.
- Stupp, R., Mason, W. P., van den Bent, J., Weller, M., Fisher, B., Taphoorn, M. J., Belanger, K., Brandes, A., Marosi, C., Bogdahn, U., Curschmann, J., Janzer, R. C., Ludwin, S. K., Gorlia, T., Allgeier, A., Lacombe, D., Cairncross, J. G., Eisenhauer, E., Mirimanoff, R. O., European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups, … National Cancer Institute of Canada Clinical Trials Group (2005). Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine, 352(10), 987–996.
- Taha, S., Abdalhamid, B. A., El-Badawy, S. A., Sorour, Y. M., Almsned, F. M., & Al-Abbadi, M. A. (2016). Expression of cytomegalovirus in glioblastoma multiforme: Myth or reality?. British journal of neurosurgery, 30(3), 307–312.
- Tang, W., Alaei-Mahabadi, B., Samuelsson, T., Lindh, M., & Larsson, E. (2013). The landscape of viral expression and host gene fusion and adaptation in human cancer. Nature communications, 4, 2513.
- Tang, K. W., Hellstrand, K., & Larsson, E. (2015). Absence of cytomegalovirus in high-coverage DNA sequencing of human glioblastoma multiforme. International journal of cancer, 136(4), 977–981.
- Trgovcich, J., Cebulla, , Zimmerman, P., & Sedmak, D. D. (2006). Human cytomegalovirus protein pp71 disrupts major histocompatibility complex class I cell surface expression. Journal of virology, 80(2), 951–963.
- Wakefield, , Pignata, A., Ghazi, A., Ashoori, A., Hegde, M., Landi, D., Gray, T., Scheurer, M. E., Chintagumpala, M., Adesina, A., Gottschalk, S., Hicks, J., Powell, S. Z., & Ahmed, N. (2015). Is CMV a target in pediatric glioblastoma? Expression of CMV proteins, pp65 and IE1-72 and CMV nucleic acids in a cohort of pediatric glioblastoma patients. Journal of neuro-oncology, 125(2), 307–315.
- Walker, D. , Shakya, R., Morrison, B., Neller, M. A., Matthews, K. K., Nicholls, J., Smith, C., & Khanna, R. (2019). Impact of pre-therapy glioblastoma multiforme microenvironment on clinical response to autologous CMV-specific T-cell therapy. Clinical & translational immunology, 8(11), e01088.
- Weathers, S. P., Penas-Prado, M., Pei, B. L., Ling, ., Kassab, C., Banerjee, P., Bdiwi, M., Shaim, , Alsuliman, A., Shanley, M., de Groot, J. F., O’Brien, B. J., Harrison, R., Majd, N., Kamiya-Matsuoka, C., Fuller, G. N., Huse, J. T., Chi, L., Rao, G., Weinberg, J. S., … Heimberger, A. B. (2020). Glioblastoma-mediated Immune Dysfunction Limits CMV-specific T Cells and Therapeutic Responses: Results from a Phase I/II Trial. Clinical cancer research : an official journal of the American Association for Cancer Research, 26(14), 3565–3577.
- Wiemels, J. , Talbäck, M., Francis, S., & Feychting, M. (2019). Early Infection with Cytomegalovirus and Risk of Childhood Hematologic Malignancies. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 28(6), 1024–1027.
- Wigertz, , Lönn, S., Hall, P., & Feychting, M. (2010). Non-participant characteristics and the association between socioeconomic factors and brain tumour risk. Journal of epidemiology and community health, 64(8), 736–743.
- Yamashita, Y., Ito, Y., Isomura, H., Takemura, , Okamoto, A., Motomura, K., Tsujiuchi, T., Natsume, A., Wakabayashi, T., Toyokuni, S., & Tsurumi, T. (2014). Lack of presence of the human cytomegalovirus in human glioblastoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc, 27(7), 922–929.
- Yang, F., Ho, H. L., Lin, S. C., Hsu, C. Y., & Ho, D. M. (2017). Detection of human cytomegalovirus in glioblastoma among Taiwanese subjects. PloS one, 12(6), e0179366.
- Yang, R., Liang, J., Xu, X., Ding, L. M., Huang, H. M., Su, Q. Z., Yan, J., & Li, Y. C. (2018). Human cytomegalovirus glycoprotein B inhibits migration of breast cancer MDA-MB-231 cells and impairs TGF-β/Smad2/3 expression. Oncology letters, 15(5), 7730–7738.
- Yang, T., Liu, D., Fang, , Ma, W., & Wang, Y. (2022). Cytomegalovirus and Glioblastoma: A Review of the Biological Associations and Therapeutic Strategies. Journal of clinical medicine, 11(17), 5221.
- Zapatka, , Borozan, I., Brewer, D. S., Iskar, M., Grundhoff, A., Alawi, M., Desai, N., Sültmann, H., Moch, H., PCAWG Pathogens, Cooper, C. S., Eils, R., Ferretti, V., Lichter, P., & PCAWG Consortium (2020). The landscape of viral associations in human cancers. Nature genetics, 52(3), 320–330.
- Zavala-Vega, , Castro-Escarpulli, G., Hernández- Santos, H., Salinas-Lara, C., Palma, I., Mejía- Aranguré, J. M., Gelista-Herrera, N., Rembao- Bojorquez, D., Ochoa, S. A., Cruz-Córdova, A., Xicohtencatl-Cortes, J., Uribe-Gutiérrez, G., & Arellano-Galindo, J. (2017). An overview of the infection of CMV, HSV 1/2 and EBV in Mexican patients with glioblastoma multiforme. Pathology, research and practice, 213(3), 271–276.
- Zhu, X., Hu, B., Hu, M., Qian, D., & Wang, B. (2020). Human cytomegalovirus infection enhances invasiveness and migration of glioblastoma cells by epithelial-to-mesenchymal transitio International journal of clinical and experimental pathology, 13(10), 2637–2647.
- Zuhair, , Smit, G. S. A., Wallis, G., Jabbar, F., Smith, C., Devleesschauwer, B., & Griffiths, P. (2019). Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta- analysis. Reviews in medical virology, 29(3), e2034.
