1. Introduction
1.1 Antimicrobial resistance
The phenomenon of antimicrobial resistance (AMR) is a natural process whereby microorganisms, such as bacteria, viruses, fungi, and parasites evolve in such a way as to withstand the action of antimicrobial agents. The pressure that antimicrobials put on pathogens is responsible for the selection of resistant strains (Fair & Tor, 2014).
With at least 700,000 deaths per year globally, AMR is recognised as a major healthcare issue rapidly spreading across the world (O’Neill, 2016). A recent Global resistance report predicts that if nothing is done, the figure might increase drastically and exceed 10 million deaths annually by 2050 (O’Neill, 2016). Infection caused by antimicrobial- resistant pathogens leads to prolonged hospital admissions and treatment failures, increasing healthcare-related costs (Prestinaci et al., 2015). Moreover, the COVID-19 pandemic has exacerbated the ongoing AMR global crisis due to the increased use of antibiotics during the treatment of COVID-19 patients, disruption of infection prevention and control practices in the overwhelming healthcare systems, and shifts of human and financial resources away from surveillance and responding to AMR threats. In addition, secondary bacterial infections caused by multidrug-resistant (MDR) bacteria have caused more COVID-19 deaths among severe and critically ill patients.
Resistance can be classified into two groups: intrinsic resistance and acquired resistance. While intrinsic resistant microorganisms refer to those that do not have the target for the antimicrobial agent, acquired resistance refers to an originally susceptible microorganism that acquires a mechanism that allows them to evade the action of the antimicrobial. Microorganisms can develop AMR either by mutations or by the acquisition of external antimicrobial resistance genes through horizontal gene transfer of mobile genetic elements (MGEs) carrying AMR genes. The latter mechanism contributes significantly to the rapid spread of resistance genes and the emergence of phenotypic drug resistance, especially in hospital settings.
1.2 Circulation of multidrug-resistant bacteria
During the last decade, healthcare professionals have started to face novel atypical challenges in the treatment of severe infections in critically ill patients due to the selection and transmission of multidrug- resistant (MDR) bacteria, i.e., strains resistant to at least one agent in three or more antimicrobial categories (Magiorakos et al., 2012). World Health Organization (WHO) published its first-ever list of antibiotic-resistant “priority pathogens” which consists of 12 bacteria that pose the greatest threat to human health (Taconelli, 2020).
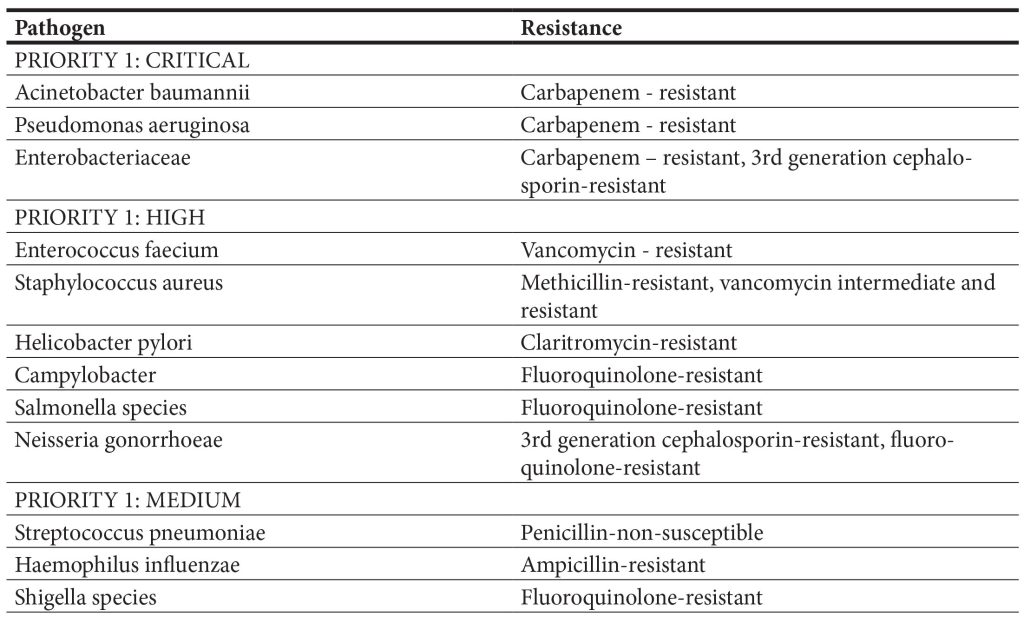
This list was created to guide and encourage re- search and development of new antibiotics as part of WHO’s efforts to combat the growing antimicrobial resistance around the world. The extensive list high- lights in particular the threat of MDR Gram-negative bacteria. Pathogens are classified as critical, high, and medium based on mortality, level of resistance, and treatability. The WHO global priority list of patho- gens ranks extended spectrum β-lactamase (ESBL) Enterobacterales, carbapenem-resistant Enterobac- teriaceae (CRE), carbapenem-resistant Pseudomo- nas aeruginosa (CRPA), and carbapenem-resistant Acinetobacter baumannii (CRAB) in the highest priority category (i.e., critical). Although several mechanisms account for the resistance to carbap- enems, the production of carbapenem-hydrolyzing enzymes encoded by horizontally transferable genes is of utmost importance. Interestingly, the Indian subcontinent and the Balkan regions are considered the main reservoirs of globally emerging New Delhi Metallo-β-Lactamases (NDM), an enzyme hydrolyz- ing a broad range of β-lactam antibiotics, including carbapenems, which are considered as the last resort antibiotics with proven efficacy to ESBL-producing Enterobacterales (Livermore et al., 2011). Important bacterial pathogens also included in the group ES- KAPEE, an acronym introduced to designate a group of bacteria that successfully escape the action of anti- biotics: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, A. baumannii, P. aeruginosa, Enterobacter species, and Escherichia coli (Ruekit et al., 2022). Although MDR bacteria are concerning causative agents of healthcare-associated infections (HCAI), reports suggest that animals, food products, and aquatic environments may also constitute signif- icant reservoirs of these bacteria and resistance genes (Wyres et al., 2018; Lepuschitz et al., 2019). The in- tensive use of antimicrobials in farming may promote the fixation of AMR genes in bacteria, which may be zoonotic or capable of transferring these genes to human-adapted pathogens or human gut microbiota via direct contact, food, or the environment. Of ma- jor concern is the mass medication of animals with antimicrobials that are critically important for hu- mans, i.e., third-generation cephalosporins and fluo- roquinolones, as well as the long-term, in-feed use of medically important antimicrobials, such as colistin and tetracyclines, for growth promotion (Argudin et al., 2017). Indeed, several studies have indicated that typical nosocomial resistances linked to ESBL and carbapenemases are emerging in Gram-negative bacteria from animals (Dahms et al., 2015; Michael et al., 2015). Also, colistin-resistant Enterobacterales, pathogens untreatable with regular antibiotics, might emerge as the result of the potential spread of plas- mid-mediated mobile colistin resistance (mcr) genes from the animal sector to humans (Liu et al., 2016). Therefore, agriculture and food-producing animals represent a significant pool of resistance genes for humans, whereas research on them should be strate- gic research Project in agriculture, food, and environ- mental microbiology, contributing to public health.
Table 1. World Health Organization priority pathogens list (Tacconelli, 2018)
Water is a crucial environmental setting and one of Earth’s most important bacterial habitats. The main sources of contamination in the aquatic environment are various genetic reactors, i.e., hu- man/animal microbiota, hospitals, large-scale ani- mal farming, wastewater, hospitals, and soil, where bacteria mix and interact through genetic exchange and recombination (Baquero et al., 2008). Surface waters, in particular, seem to play a key role in this spread, serving both as habitats and as transport sys- tems for MDR bacteria (Falgenhauer et al., 2019). To date, many studies have highlighted wastewater as a hotspot for the dissemination of ARGs into the envi- ronment (Zhang et al., 2015; Gundogdu et al., 2013; Mokracka et al., 2012). Another important public health concern is the potential effect of antibiotics in the aquatic environment regarding their potential influence on potable water quality and global human health. The significant existence of antibiotics in the environment, especially in aquatic ecosystems, can be attributed to multiple factors, including the pro- duction of active pharmaceutical ingredients, the excretion of residues after usage, and the discarding of unused medicines from medicinal practices, labo- ratories, factories, residential, and commercial insti- tutes (Kemper, 2008). As a consequence, aquatic bi- ota became exposed to unprecedented antibiotic se- lection pressures, contributing to the rapid resistance development we face today. Furthermore, antibiotic traces in potable water and food can lead to antibiot- ic-induced microbiome depletion and selective pres- sure on the human microbiome, which might serve as a reservoir for ARGs. By horizontal gene transfer, such resistance determinants may be transferred to pathogenic bacteria compromising antibiotic treat- ment and the outcome. Although previous scientific literature reports the presence of antibiotics in sur- face waters and studies focused on genes involved in AMR, mechanisms leading to the selection and acquisition of resistance determinants by bacterial strains, as well as ARG maintenance in aquatic envi- ronments, remain unclear. At a policy level, no safety threshold values or standardised guidelines on the environmental risk assessment of antibiotics current- ly exist, which is largely due to insufficient data on environmentally relevant concentrations (Niegows- ka et al., 2021). At the European level, the amended Water Framework Directive includes the Watch List program, which aims to gather good data quality for substances contaminating waterbodies to determine the risk they may pose to aquatic environments (Nie- gowska et al., 2021).
Antimicrobial resistance in humans, animals, and the environment is depicted in Figure 2.
1.3 Antimicrobials use in humans, animals, and plants
Although ARGs existed in nature before the discovery of antibiotics, the emergence and spread of AMR in pathogenic strains occurred in response to the development and use of these agents. Cumulating reports indicate that antimicrobial misuse and abuse in human, animal, and environmental sectors, as well as the spread of resistant bacteria and resistance determinants within and between these sectors, are the main drivers of AMR (Holmes et al., 2015).
Antimicrobials are used for various purposes (therapeutic, prophylactic, and development promoters) and play an important role in animal production. Some antimicrobials used in humans and animals (tetracycline, triazoles, and streptomycin) are used for prophylaxis and treatment against bacteria that cause plant infections (Sundin et al., 2018). There are some classes of antimicrobials for human use only (carbapenems) and others for animal use only (flavophospholipol and ionophores) (Wall et al., 2016; Van Boeckel et al., 2015). The application of antibiotics in veterinary settings is different between food-producing animals and pets, where the prescription of antibiotics is generally comparable to those in humans (McEwen & Collignon, 2018). Antibiotics in poultry are often administered to the whole group through water or feed without any clinical indications for preventive purposes.
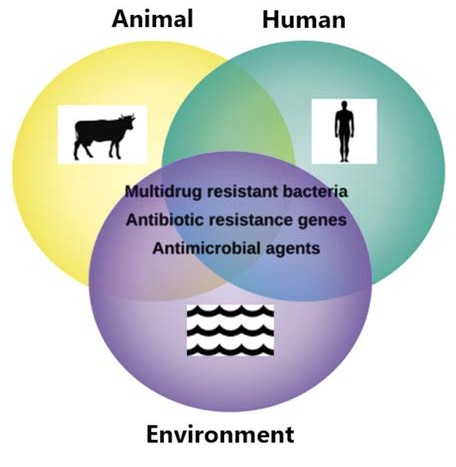
Figure 2. Antimicrobial resistance in humans, animals and environment
Some antibiotics were used for decades before resistance developed, while others developed resistance in a much shorter period of time. Antibiotics with a slow development of resistance, especially vancomycin, are valued for their enduring ability to treat infections.
In 2019, the WHO identified 32 antimicrobial agents in hospital development, of which only six were classified as innovative. The world’s health systems are being impacted by the lack of antimicrobials. Accordingly, antibiotics play an important role in controlling bacterial pathogens, but decreasing their usage is vital because of the emergence of resistant pathogens and transmissible ARGs. Recent studies have enabled us to understand the gene exchange and pathogen interactions in various One Health niches, which is important for the development of alternative antibiotic approaches, such as the use of bacteriophages. Additionally, vaccines in both human and veterinary medicines, also play an important role in controlling ABR as they reduce the need for antibiotic therapy.
2. One Health approach to combat antimicrobial resistance
2.1 Historical perspective of One health concept
One Health is an approach that acknowledges that human health is closely connected to the health of animals and our shared environment. Although the phrase “One Health” is relatively new, the concept has long been recognized on a national and international level. The pioneers in this field include the following persons:
- Rudolf Virchow recognized the link between human and animal health and introduced the term “zoonosis,” which encompasses the relationship between human and animal healt
- William Osler had a deep interest in the linkages between human and veterinary medicine
- James Steele was responsible for the official inclusion of veterinarians into the US Public Health Service and in 1947, he founded the Veterinary Public Health Division at CDC, which led to rapid advances in the control and prevention of zoonotic diseases both in the United States and internationally.
- Calvin Schwabe coined the term “One Medicine” and spent a lifetime practicing and teaching the principles of One Health (Schwabe, 1964).
2.2 A modern perspective of One Health
On September 29, 2004, the Wildlife Conservation Society published the 12 Manhattan principles and formed the basis of the “One Health, One World” concept. In 2007, the One Health approach is recommended for pandemic preparedness. One Health becomes a recommended approach and a political reality in 2008.
Although the epidemiology of AMR at the human-animal-water interface is invariably complex, ARGs’ diversity and the evolvability of antibiotic resistance particularly depend on control of the flow of active antimicrobials, bacterial populations, and genetically based biological information along different genetic reactors, i.e., hospital settings, farm animals, and water sources. Due to the multidisciplinary nature of the issue, an integrative “One Health” concept is best suited to address the existing and emerging threats of AMR as well as to understand how human behaviour, environmental degradation, and antibacterial usage in animals lead to AMR transmission (One Health Initiative, 2017). However, the interdisciplinary, intersectoral, and multi-institutional “One Health“ approach to fight clinically-relevant MDR organisms, has not been effectively implemented in many countries. The understanding of genetic relatedness between MDR organisms from hospital settings, animal and water sources, will contribute to the further strengthening of the national control of drug-resistant diseases. In light of that, it is known that the distribution of resistant bacteria in clinical settings is acceptably well-documented on a global scale. Contrary to this, the distribution and evidence of MDR bacteria in the environment are hardly based on qualitative data.
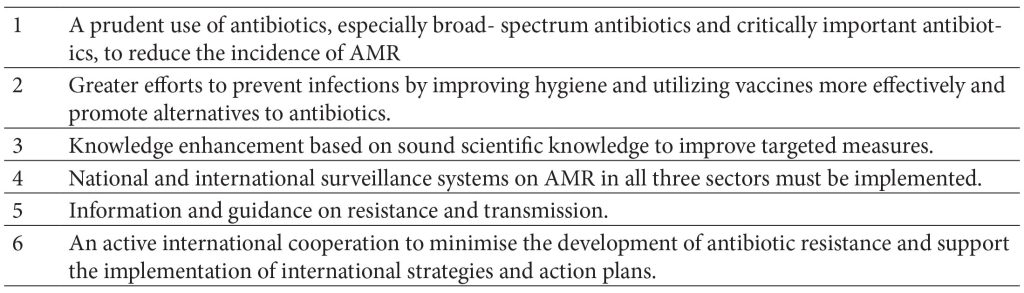
Table 2. Goals of the national strategies concerning antimicrobial resistance
2.3 Genomic approach
Traditional microbiological methods such as culturing bacterial pathogens and sequencing isolates require additional logistics and may not be practical in the field. Therefore, The Global Antimicrobial Resistance and Use Surveillance System (GLASS) established by the WHO strongly suggests using whole-genome sequencing (WGS) for surveillance of AMR to add important, policy-relevant information for AMR surveillance, to better inform strategies to tackle AMR, and to enhance the overall surveillance capacity (WHO, 2020).
Implementation of the “One health genomics“, as a novel concept to tackle AMR, could provide an in- depth genomic insight into the AMR landscape of a region, microbial composition across different sam- ple sources, the genetic relatedness of the MDR bacteria, and differences in resistome profiles of interconnected sectors. State-of-the-art technolo- gies, such as a sequence-based approach to studying genomes from a mixed microbial community known as metagenomics is being applied to understand and decipher the complexity of AMR in different nich- es of One health sector. Moreover, an integrative genomic approach enables scientists to elucidate hotspots of AMR and transmission routes of ARGs, the ARGs diversity, as well as the relatedness between MDR microorganisms from different environmental compartments, which could enable the detection of the most important environmental point sources of ARGs. A novel genomic approach could serve as the most optimal tool to achieve the above-mentioned goals.
3. Conclusion
In conclusion, supporting and implementing a “one health” approach is critical to combating AMR. This necessitates accelerating global development, innovating to ensure the future, collaborating for more effective action, investing in long-term solutions, and enhancing global governance and accountability. The rise of AMR can be reduced when antibiotics are used only for treatment, rarely for prophylaxis, and never as growth promoters. Success will require strict and efficient control of the types and amounts of antimicrobials used, monitoring, reporting, and controlling the proliferation of resistant microorganisms that spread to the environment.
Funding: This research received no external funding.
Informed Consent Statement: Not applicable.
Data Availability Statement: Not applicable.
Conflicts of Interest: The author declares no conflicts of interest.
References
- Fair, R. J., & Tor, Y. (2014). Antibiotics and bacterial resistance in the 21st century. Perspectives in medicinal chemistry, 6, 25–64.
- O’Neill, J. (2016). Tackling drug-resistant infections globally: Final report and recommendations. London: HM Government and the Wellcome Trus
- Prestinaci, F., Pezzotti, P., & Pantosti, A. (2015). Antimicrobial resistance: a global multifaceted phenomeno Pathogens and global health, 109(7), 309–318.
- Magiorakos, P., Srinivasan, A., Carey, R. B., Carmeli, Y., Falagas, M. E., Giske, C. G., Harbarth, S., Hindler, J. F., Kahlmeter, G., Olsson-Liljequist, B., Paterson, D. L., Rice, L. B., Stelling, J., Struelens, M. J., Vatopoulos, A., Weber, J. T., & Monnet, D. L. (2012). Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases, 18(3), 268–281.
- Tacconelli, , Carrara, E., Savoldi, A., Harbarth, S., Mendelson, M., Monnet, D. L., Pulcini, C., Kahlmeter, G., Kluytmans, J., Carmeli, Y., Ouellette, M., Outterson, K., Patel, J., Cavaleri, M., Cox, E. M., Houchens, C. R., Grayson, M. L., Hansen, P., Singh, N., Theuretzbacher, U., … WHO Pathogens Priority List Working Group (2018). Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. The Lancet. Infectious diseases, 18(3), 318–327.
- Livermore, D. , Walsh, T. R., Toleman, M., & Woodford, N. (2011). Balkan NDM-1: escape or transplant?. The Lancet. Infectious diseases, 11(3), 164.
- Ruekit, , Srijan, A., Serichantalergs, O., Margulieux, K. R., Mc Gann, P., Mills, E. G., Stribling, W. C., Pimsawat, T., Kormanee, R., Nakornchai, S., Sakdinava, C., Sukhchat, P., Wojnarski, M., Demons, S. T., Crawford, J. M., Lertsethtakarn, P., & Swierczewski, B. E. (2022). Molecular characterization of multidrug- resistant ESKAPEE pathogens from clinical samples in Chonburi, Thailand (2017-2018). BMC infectious diseases, 22(1), 695.
- Wyres, L., & Holt, K. E. (2018). Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Current opinion in microbiology, 45, 131–139.
- Lepuschitz, , Schill, S., Stoeger, A., Pekard- Amenitsch, S., Huhulescu, S., Inreiter, N., Hartl, R., Kerschner, H., Sorschag, S., Springer, B., Brisse, S., Allerberger, F., Mach, R. L., & Ruppitsch, W. (2019). Whole genome sequencing reveals resemblance between ESBL-producing and carbapenem resistant Klebsiella pneumoniae isolates from Austrian rivers and clinical isolates from hospitals. The Science of the total environment, 662, 227–235.
- Argudín, A., Deplano, A., Meghraoui, A., Dodémont, M., Heinrichs, A., Denis, O., Nonhoff, C., & Roisin, S. (2017). Bacteria from Animals as a Pool of Antimicrobial Resistance Genes. Antibiotics (Basel, Switzerland), 6(2), 12.
- Dahms, , Hübner, N. O., Kossow, A., Mellmann, A., Dittmann, K., & Kramer, A. (2015). Occurrence of ESBL-Producing Escherichia coli in Livestock and Farm Workers in Mecklenburg-Western Pomerania, Germany. PloS one, 10(11), e0143326.
- Michael, B., Freitag, C., Wendlandt, S., Eidam, C., Feßler, A. T., Lopes, G. V., Kadlec, K., & Schwarz, S. (2015). Emerging issues in antimicrobial resistance of bacteria from food-producing animals. Future microbiology, 10(3), 427–443.
- Liu, Y. Y., Wang, Y., Walsh, T. R., Yi, X., Zhang, R., Spencer, J., Doi, Y., Tian, G., Dong, B., Huang, X., Yu, L. F., Gu, D., Ren, H., Chen, X., Lv, L., He, D., Zhou, H., Liang, Z., Liu, J. H., & Shen, J. (2016). Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. The Lancet Infectious diseases, 16(2), 161–168.
- Baquero, F., Martínez, J. L., & Cantón, R. (2008). Antibiotics and antibiotic resistance in water environmen Current opinion in biotechnology, 19(3), 260–265.
- Falgenhauer, , Schwengers, O., Schmiedel, J., Baars, C., Lambrecht, O., Heß, S., Berendonk, T. U., Falgenhauer, J., Chakraborty, T., & Imirzalioglu, C. (2019). Multidrug-Resistant and Clinically Relevant Gram-Negative Bacteria Are Present in German Surface Waters. Frontiers in microbiology, 10, 2779.
- Zhang, , Han, B., Gu, J., Wang, C., Wang, P., Ma, Y., Cao, J., & He, Z. (2015). Fate of antibiotic resistant cultivable heterotrophic bacteria and antibiotic resistance genes in wastewater treatment processes. Chemosphere, 135, 138–145.
- Gündoğdu, , Jennison, A. V., Smith, H. V., Stratton, H., & Katouli, M. (2013). Extended-spectrum β-lactamase producing Escherichia coli in hospital wastewaters and sewage treatment plants in Queensland, Australia. Canadian journal of microbiology, 59(11), 737–745.
- Mokracka, J., Koczura, R., & Kaznowski, A. (2012). Multiresistant Enterobacteriaceae with class 1 and class 2 integrons in a municipal wastewater treatment plan Water research, 46(10), 3353–3363.
- Kemper, (2008). Veterinary antibiotics in the aquatic and terrestrial environment. Ecological Indicators, 8(1), 1-13.
- Niegowska, , Sanseverino, I., Navarro, A., & Lettieri, T. (2021). Knowledge gaps in the assessment of antimicrobial resistance in surface waters. FEMS microbiology ecology, 97(11), fiab140.
- Holmes, H., Moore, L. S., Sundsfjord, A., Steinbakk, M., Regmi, S., Karkey, A., Guerin, P. J., & Piddock, L. J. (2016). Understanding the mechanisms and drivers of antimicrobial resistance. Lancet (London, England), 387(10014), 176–187.
- Sundin, W., & Wang, N. (2018). Antibiotic Resistance in Plant-Pathogenic Bacteria. Annual review of phytopathology, 56, 161–180.
- Wall, B. , Mateus, A., Marshall, L., Pfeiffer, D. U., Lubroth, J., Ormel, H. J., Otto, P., & Patriarchi, (2016). Drivers, dynamics and epidemiology of antimicrobial resistance in animal production.
- Van Boeckel, T. P., Brower, , Gilbert, M., Grenfell, B. T., Levin, S. A., Robinson, T. P., Teillant, A., & Laxminarayan, R. (2015). Global trends in antimicrobial use in food animals. Proceedings of theNational Academy of Sciences of the United States of America, 112(18), 5649–5654.
- McEwen, A., & Collignon, P. J. (2018). Antimicrobial Resistance: a One Health Perspective. Microbiology spectrum, 6(2), 10.1128/microbiolspec.ARBA-0009-2017.
- Schwabe, W. (1964). Veterinary Medicine and Human Health (1st ed.). Baltimore: Williams and Wilkins.
- One Health Initiative (n.d.). One Health Initiative will unite human and veterinary medicine.
- World Health Organisation (n.d.). Global Antimicrobial Resistance and Use Surveillance System (GLASS) Whole- genome sequencing for surveillance of antimicrobial resistance. Who.in https://www.who.int/docs/default- source/antimicrobial-resistance/glass_wgs_report_ v8_web.pdf?sfvrsn=9ef1b4a5_1
