Abstract: Viral infections such as Epstein-Barr virus (EBV) and Herpes simplex virus (HSV) represent significant complications in patients with haematological malignancies and increase the frequency of morbidity and mortality. These infections represent one of the main factors of unsuccessful treatment; therefore, regular monitoring of active viral infections is necessary. The work aimed to determine the frequency and dynamics of active EBV and HSV infection in patients with haematological malignancies, monitored at the Institute of Microbiology and Immunology, Faculty of Medicine University of Belgrade over two years. This study included 35 patients treated at Hematology Clinics of the University Clinical Center of Serbia. Patients were monitored during 2020-2021. Ten blood samples were taken from each patient during monitored period. The samples were taken in a time interval between 2 weeks and three months, depending on the arrival of patients at medical controls. The first sample is marked with the first measurement of blood drawn during 2020 for each patient. The second measurement was a second blood sample taken in the previously mentioned time interval, and so on up to the tenth sample marked as the tenth measurement. For the detection of EBV DNA, the “in-house” PCR method was used to detect the EBNA-1 gene. According to the manufacturer’s instructions, the commercial kit Ampli Sens® HSVI, II-FRT was used for HSV DNA detection (InterLabService, Moscow, Russia). The lowest frequency of EBV infection was during the second measurement (8.57%), while the highest was during the eighth and tenth measurements (31.43%). The highest frequency of HSV infection was during the eighth measurement (5.71%), while during the third, fifth and ninth measurements, none of the subjects had positive PCR for HSV DNA. A statistically significant difference was found in the frequency of EBV infection in later measurements compared to earlier measurements (p=0.002). In contrast, this was not the case in the study of HSV frequency infection (p=0.750). This study presents the dynamics of the frequency of active EBV and HSV infection in patients with haematological malignancies in two-year period. The frequency of active EBV infection was significantly higher compared to HSV infection.
Keywords: Hematological malignancies; PCR; EBV; HSV; frequency of active infection
1. Introduction
Haematological malignancies are diseases characterised by abnormal blood cell production and function (Auberger et al. 2020). Three main groups of haematological malignant diseases are leukemia, lymphomas and multiple myeloma (Nedeljkov et al. 2020). They account for 1.2 million new cases each year worldwide and represent about 7% of all newly diagnosed cancers (Auberger et al. 2020).
Leukaemia is a group of hematologic cancers that arise in the blood-forming cells of the bone marrow and lead to the accumulation of abnormal blood cells in the bone marrow (BM) and bloodstream. While all age groups can be affected, leukaemia is the most common pediatric tumour (Auberger et al. 2020). There are four main types of leukaemia: Acute lymphocytic (or lymphoblastic) leukaemia (ALL), Acute myeloid leukaemia (AML), Chronic lymphocytic leukaemia (CLL) and Chronic myeloid leukaemia (CML) (Nedeljkov et al. 2020; Aster et al. 2010). Lymphomas begin in cells of the lymph system – T and B lymphocytes. Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) are the two main types (Nedeljkov et al. 2020).
The most important etiological factors contributing to haematological malignancies are ionising radiation, chemical (benzene), genetic factors, chromosomal aberrations and oncogenic viruses (Nedeljkov et al. 2020). Viral infections are one of the most important causes of morbidity and mortality in patients with haematological malignancies, among which Epstein-Barr virus (EBV), herpes simplex virus (HSV), cytomegalovirus (CMV), varicella-zoster virus (VZV) and respiratory syncytial virus (RSV) (Marmont et al. 1991; Wade et al. 2006) are the most common.
EBV is highly prevalent since it affects 80-90% of individuals worldwide. EBV causes three types of infection: lytic in permissive cells (epithelial cells of the oropharynx and B cells), latent infection in non-permissive cells – memory B lymphocytes and transformation of B lymphocytes. EBV causes lifelong latent infection in memory B cells, and after their activation, reactivation occurs, which is always accompanied by virus excretion in saliva, but with no clinical manifestations, except in immunocompromised persons (Ćupić et al. 2019).
HSV is the first recognised human herpesvirus. There are two types of human herpesvirus – HSV 1 and 2. HSV 1 is associated with infections such as gingivostomatitis, labial herpes and keratoconjunctivitis, while HSV 2 is mainly associated with genital herpes (Ćupić et al. 2019).
EBV, the first identified human oncogenic virus, contributes to approximately 1.5% of malignant tumours worldwide. EBV has a high oncogenic potential and is associated with developing certain human tumours such as lymphomas, nasopharyngeal cancer and lymphoproliferative diseases in people with immunodeficiencies (Yuan et al. 2022). In patients with CLL, HSV reactivation was found in 15%, while among patients with acute leukaemia, it was found in 90% (Wade et al. 2006; Ljungman et al. 2004; Sandherr et al. 2006). HSV infections in immunocompromised patients are more invasive than in immunocompetent. Changes in the skin and mucous membranes heal slower, they are associated with slower shedding of the virus, and are often disseminated (Wade et al. 2006).
Despite the use of various antiviral drugs in clinical practice, infections-caused agents such as HSV, VZV, EBV and CMV are associated with the most frequent and severe complications in patients with haematological malignancies. These infections increase morbidity and mortality and are the main factor of unsuccessful treatment. Therefore, constant laboratory monitoring of the reactivation of latent viral infections in these patients is necessary (Busca et al. 2012).
The work aimed to determine the frequency and dynamics of active EBV and HSV infection of patients with haematological malignancies, which were monitored in the Virology Laboratory of the Institute of Microbiology and Immunology, Faculty of Medicine University of Belgrade over two years.
2. Results
2.1. Patients
In this study, 35 patients were included. The average age of the patients was 39.43 (20-65) years. Twenty-three (65.71%) patients were male, while twelve were female (34.29%). The most common diagnosis was AML 16/35 (45.71%), then lymphoid cell leukaemia 5/35 (14.29%), myelodysplastic syndrome 4/35 (11.43%) and myeloid leukaemia 4/35 (11.43%), CLL 2/35 (5.71%). On the other hand, the diagnoses are ALL, peripheral T-cell lymphoma, HL and post-transplant condition organs/tissues were present in 1 patient each (2.86%).
2.2. Frequency of active EBV and HSV infection
The lowest positive rate of active EBV infection was during the second measurement (8.57%) while the highest during the eighth and tenth measurements (31.43%) (Figure I). A statistically significant difference was found in the frequency of active EBV infection in later measurements compared to earlier measurements (p=0.002).

Figure 1. Percentage of active EBV and HSV infection at each measurement in 35 patients suffering from haematological malignancies
The frequency of HSV active infection was significantly lower than active EBV infection in the same patients. None of the patients had an active HSV infection during the third, fifth and ninth measurements. The highest frequency was during the eighth measurement (5.71%) (Figure I). A statistically significant difference in the frequency of active HSV infection in later measurements has not been established compared to earlier measurements (p=0.750).
2.3. Frequency of active EBV and HSV infection in relation to gender
The frequency of active EBV infection in male patients has increased over time. The lowest frequency of active EBV infection in male patients was present during the first measurement (8.57%), while the highest frequency of active EBV infection in male patients was during the last measurement (31.43%) (Figure II). A statistically significant difference was found in the frequency of active EBV infection in male patients in later measurements compared to earlier measurements (p=0.004).
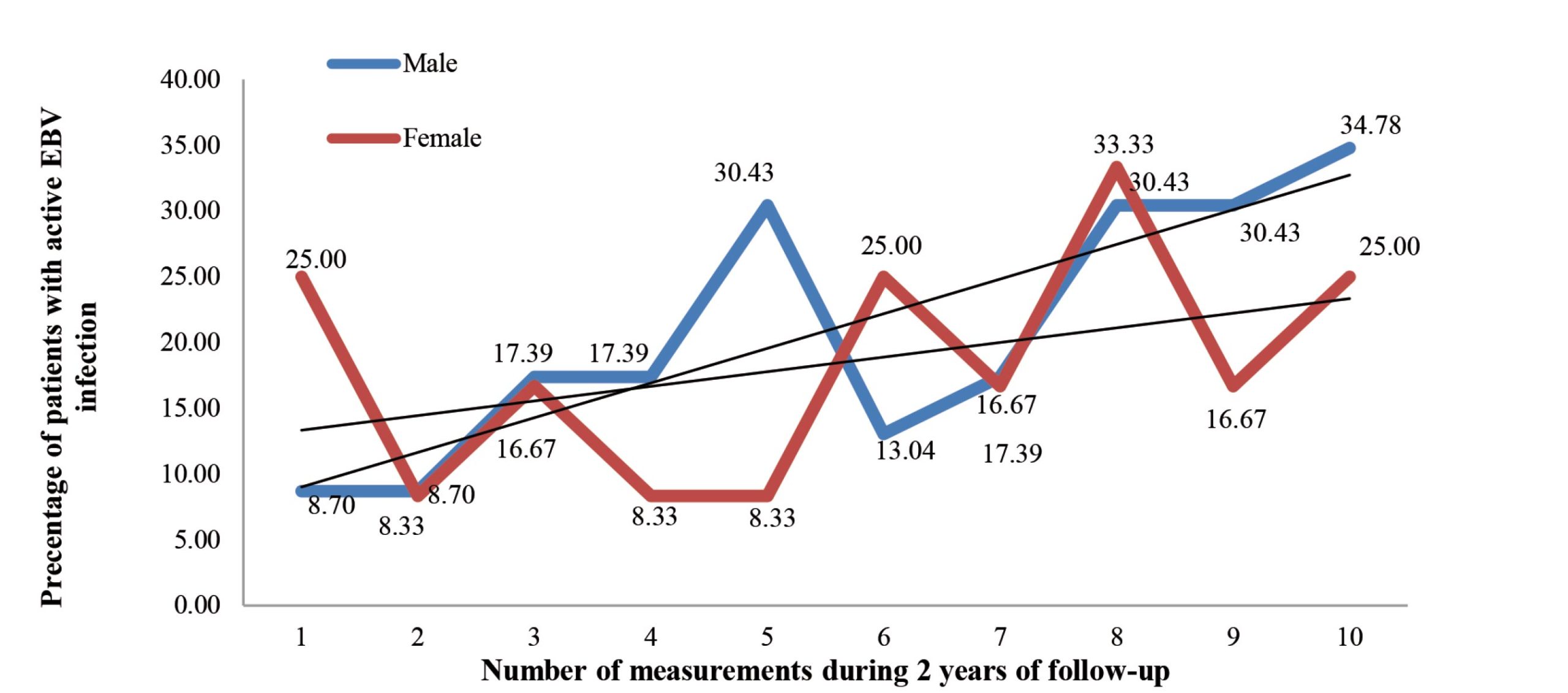
Figure 2. Percentage of active EBV infection in each measurement in 35 patients suffering from haematological malignancies in relation to gender
The frequency of active EBV infection in female patients had similar dynamics during ten measurements, while no statistically significant difference was found in the later measurements in the relationship to earlier measurements (p=0.264) (Figure II).
None of the male patients had an active HSV infection in most measurements (first, second, third, fifth, seventh and ninth). In the fourth, sixth and tenth measurements, the frequency was 4.35%, while the highest frequency of active HSV infection in male patients was in the eighth measurement (8.70%) (Figure III). No statistically significant difference was found in the frequency of active HSV infection among male patients during the later compared to the earlier measurements (p=0.202).
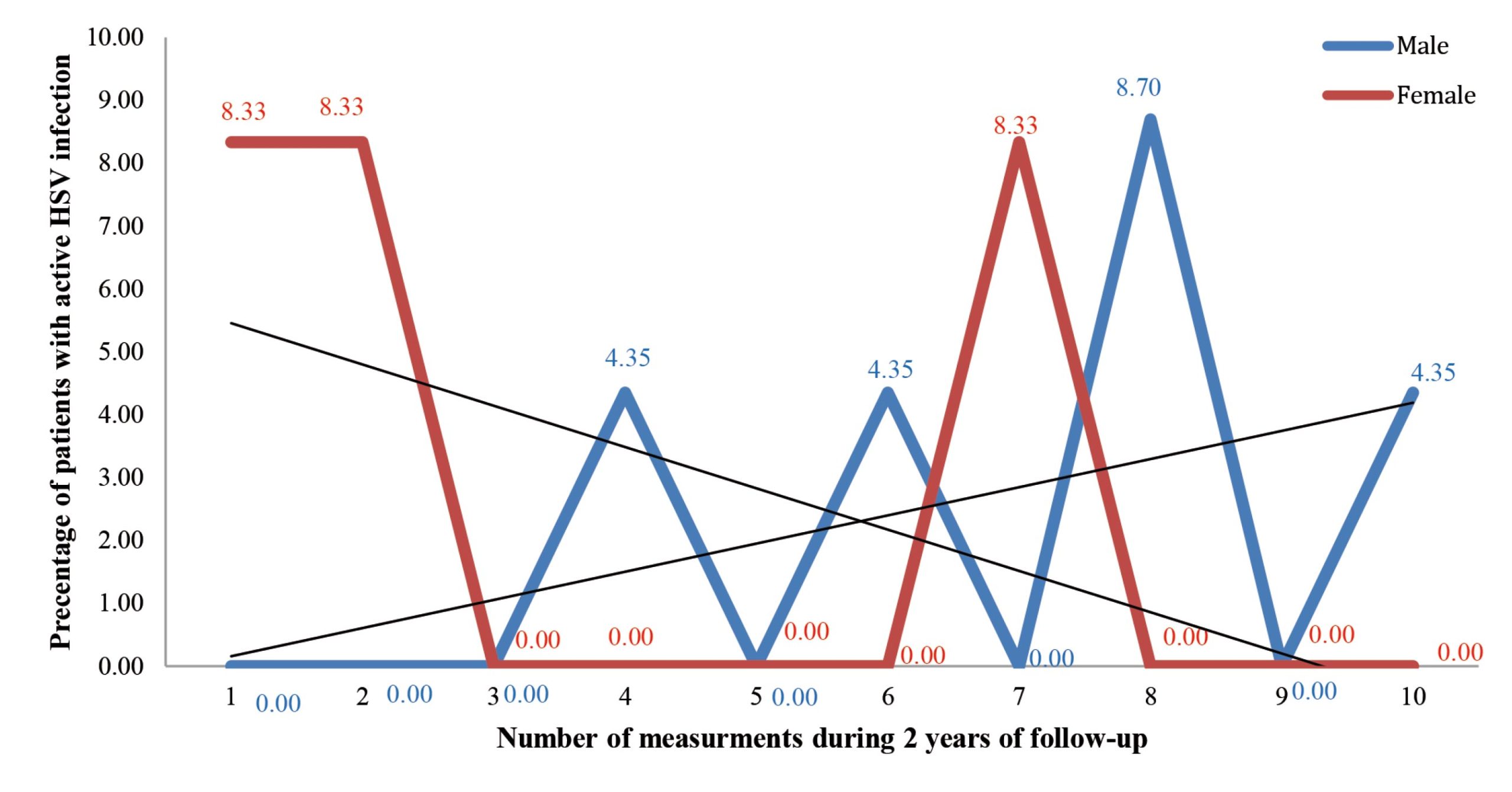
Figure 3. Percentage of active HSV infection in each measurement in 35 patients suffering from haematological malignancies in relation to genderSa me anu et, vem in nicatin virit; hoccion fessimedi
None of the female subjects had an active HSV infection in the largest number of measurements (third, fourth, fifth, sixth, eighth, ninth and tenth), while the frequency in the first, second and seventh measurements was 8.33% (Figure III). No statistically significant difference was found in the frequency of active HSV infection among female patients during the later compared to the earlier measurements (p=0.147).
3. Discussion
Viral infections represent one of the most critical factors in the unsuccessful treatment of patients with haematological malignancies (Busca et al. 2012). EBV and HSV infections occur because of compromised immune response. The immune response is compromised in these patients because of the disease’s characteristics and side effects of the applied chemotherapy (Hallek et al. 2018; Andrei et al. 2021). Infections caused by viral agents are associated with the most frequent and severe complications in patients with haematological malignancies. They lead to increased morbidity and mortality and are the main factor of unsuccessful treatment. Therefore, constant laboratory monitoring of the reactivation of latent viral infections in these patients is necessary (Busca et al. 2012). Active EBV infection in patients with NHL is variable and is between 0.1% and 63%, while in patients suffering from Burkitt’s lymphoma, the most aggressive type of NHL in endemic areas is 100% (Ma et al. 2011; Ru et al. 2022). Five-year survival in patients with HL, over 45 years of age with active EBV infection was 37%, while of patients who did not have an active EBV infection was 74% (Liu et al. 2010). In our study, the highest frequency of active EBV infection was 31.43%, and the lowest 8.57%. This study determined EBV PCR positivity throughout 10 measurements, and it was possible to monitor the dynamics and change of EBV positivity over time. This represents the main advantage of this study compared to many others that conducted only one measurement (Guan et al. 2017; Ahmed et al. 2012). In this study, the frequency of active EBV infection in patients with haematological malignancies increased during the follow-up period, and the frequency of EBV in male respondents increased over time, while it did not change significantly among women. Guan et al. (2017) examined the rate of EBV reactivation in patients with acute leukaemia in the period of 2-5 years from the diagnosis and found it in 34.6% of patients with acute leukaemia, while in healthy controls it was significantly smaller around 5.4%. Ahmed et al. (2012) conducted a study examining the incidence of EBV infection and found that EBV reactivation in patients with different types of leukaemia was 36.3%, while EBV reactivation was not observed in the control group. Ru et al. (2022) found that the one-year cumulative incidence of EBV in patients with ALL was 23% and in AML patients 28%. The percentage of EBV infection in the mentioned studies is similar to later measurements in our study. However, to make an adequate comparison with our study, data on the dynamics and changes in the frequency of active infection during a period are necessary.
In our study highest frequency of active HSV infection was 5.71%, while none of the patients had an active HSV infection in several measurements. It was found that there were no significant changes in the frequency of active HSV infection over time, as well as in relation to gender. Previous studies showed that in patients with CLL, reactivation of HSV was found in 15% of patients, while among patients with acute leukaemias, it was found in 90% of patients (Ru et al. 2022; Lungman et al. 2004; Sandherr et al. 2006). The incidence of reactivation of HSV infection in patients with acute leukaemia is high and present in 37-68% of patients treated with intensive chemotherapy (Djuric et al. 2009; Yahav et al. 2009). Hong et al. (2020) say that patients suffering from acute leukaemias receiving intensive chemotherapy need prophylactic application of antiviral drugs effective in suppressing HSV infection, such as acyclovir and valacyclovir. Park et al. (2011) reported that HSV reactivation was 10.7% in patients with diffuse large B-cell lymphoma. The cumulative incidence of HSV reactivation during five years amounted to 20.16% in subjects suffering from NHL and HL who were in intensive care chemotherapy without prophylactic antiviral therapy (Lee et al. 2012). The results from the mentioned research are not in according to the results of this study because the frequency of HSV infection is significantly higher than in our study. The discrepancy can be explained by the fact that in the mentioned studies, patients were treated with intensive chemotherapy, which can cause side effects, at the same time, there is a possibility that the patients from our study were on prophylactic treatment antiviral therapy, such as acyclovir. Also, in all previously mentioned studies only detection of active HSV infection was done in one time so we could not compare changes in HSV positivity over time.
In 9 patients, active EBV infection was not found in any of the ten measurements, while in 26 patients, active HSV infection was not found in any of the ten measurements. In one patient, active EBV and SBV infections were not found during all ten measurements, and his diagnosis was post-organ/tissue transplant condition.
The main limitation of this study is the lack of exact time of diagnosis and the impossibility of comparing the measurements of active EBV and HSV infections in relation to the time of diagnosis. Other limitations are the lack of a control group and the low number of patients.
4. Materials and Methods
Thirty five patients treated at Hematology Clinics of the University Clinical Center of Serbia were included in this retrospective study conducted at the Institute of Microbiology and Immunology, Faculty of Medicine, University of Belgrade. Patients were monitored during 2020 and 2021. Ten blood samples were taken from each patient individually during the two-year period. Blood samples are taken at time intervals ranging between 2 weeks and 3 months, depending on the arrival of patients at medical controls. The first blood sample taken in 2020 for each patient was marked as the first measurement. The second measurement was the second blood sample taken in the previously mentioned time interval; the third blood sample was the third measurement until the tenth sample was marked as the tenth measurement. For each patient from the Virology Laboratory’s documentation, the Polymerase chain reaction (PCR) results for EBV and HSV for all ten blood samples, together with anamnestic data on sex, year of birth and disease diagnosis, were taken.
4.1. Nucleic acid extraction
Each blood sample was centrifuged for 5-10 minutes at 2000xg. Then, according to the manufacturer’s instructions, 200µl of buffy coat was used for nucleic acid extraction using a commercial kit – QIAmp DNA Mini Kit (Qiagen, Valencia, CA, USA).
4.2. Detection of EBV DNA
For the detection of EBV DNA, the “in-house” nested PCR method for detecting the EBNA-1 gene was used. Primers for the first PCR round of EBV detection were: Fw 5’ – AGATGGTGAGCCTGACGTG – 3’ and Rev 5’ – GCATCCTTCAAAACCTCAGC – 3’, while for the second round Fw 5’ -GCATCCTTCAAAACCTCAGC – 3’ and Rev 5’ – GGGTCCAGGGGCCATTCCAAA – 3’. The expected length amplified EBNA1 PCR product was 329 bp (Lorenzetti et al. 2010). The total volume of the PCR reaction for the detection of EBV DNA was 25 µl. The Taq DNA Polymerase kit (Qiagen, Germany) was used for the PCR mix. PCR program for the first EBV amplification round included denaturation at 95°C for 10 minutes, then 40 cycles of the following three steps: denaturation at 95°C for 2 minutes, primers annealing at 58°C for 1 minute and elongation at 72°C for 1 minute; finally, the final extension was done at 72°C on the 10 minutes. Gel electrophoresis in 2% agarose gel was used to visualise EBV PCR products. The appropriate size of the positive EBV PCR product was 329bp.
4.3. Detection of HSV DNA
According to the manufacturer’s instructions, the commercial kit AmpliSens® HSV I, II-FRT was used for HSV DNA detection (InterLabService, Moscow, Russia). AmpliSens® HSV I, II-FRT PCR, the kit is an in vitro nucleic acid amplification test for the qualitative detection of DNA HSV 1 and 2 in clinical material. Detection of HSV 1 and 2 by the PCR method is based on amplification of specific regions of the virus genome using specific HSV 1 and 2 primers. The target gene detected is the gpB gene. Detection of HSV 1 and 2 by this method involves the extraction of DNA HSV 1 and 2 from clinical materials with the presence of internal control (IC) and real-time PCR amplification of DNA HSV 1 and 2 and IC. The total volume of the PCR reaction for HSV DNA detection was 30 µl. PCR amplification program for HSV included following steps: denaturation at 95°C for 15 minutes, then five cycles of: denaturation at 95°C for 5 seconds, annealing at 60°C for 20 seconds and extension at 72°C for 15 seconds, then followed by 40 cycles of denaturation at 95°C for 5 seconds, annealing at 60°C for 30 seconds (fluorescent signal) and extension at 72°C for 15 seconds.
4.4. Statistical analysis
The data were analysed using descriptive and analytical statistics methods in the computer program “Statistical Package for the Social Sciences version 26” for Windows. To describe categorical data, absolute and relative numbers (in the form of a percentage) were used. Numeric variables are defined by median and range values. The normality of the distribution was evaluated mathematically (Shapiro-Willk and Kolmogorov-Smirnov tests, skewness and kurtosis measure symmetry and peaking of the distribution and coefficient of variation) and graphically (histogram and diagram boxes). Linear regression was used to examine the trend of changes in the percentage representation of positive findings of the studied viruses, showing the linear function equation and the p-value (equation in the form: y=a+bx) and coefficient of determination (R2). The probability value of p≤0.05 is considered statistically significant in all statistical tests.
5. Conclusions
This study presents the dynamics of the frequency of active EBV and HSV infection in patients with haematological malignancies in two years. The frequency of active EBV infection was significantly higher than that of active HSV infection. The importance of monitoring active EBV infection in patients with malignancies is highlighted because this virus has a high oncogenic potential and is associated with lymphoma, nasopharyngeal cancer and lymphoproliferative disease in persons with immunodeficiencies. So, the presence of one malignant tumour can encourage the emergence of a new one due to frequent active EBV infection.
Funding: This research received no external funding.
Informed Consent Statement: Not applicable.
Data Availability Statement: All data are presented in the article.
Conflicts of Interest: The authors declare no conflicts of interest.
References
- Auberger, P., Tamburini-Bonnefoy, J., & Puissant, A. (2020). Drug Resistance in Hematological Malignancies. International journal of molecular sciences, 21(17), 6091.
- Nedeljkov V. (2020). Patofiziologija krvi i limfoidnog tkiva. In: Nešović Ostojić J, Radosavljević S. T, Pešić Č. B. (ured.) (2020). Mehanizmi poremećaja zdravlja, drugi deo (222-226). Beograd: Medicinski fakultet Univerziteta u Beogradu, CIBID.
- Aster J. (2010). Hematopoetski i limfni sistem. In: Boričić, I, Đuričić, S. (ured.) (2010). Robinsove osnove patologije (421-467) Beograd: Data Status.
- Marmont, A. M., Horowitz, M. M., Gale, R. P., Sobocinski, K., Ash, R. C., van Bekkum, D. W., Champlin, R. E., Dicke, K. A., Goldman, J. M., & Good, R. A. (1991). T-cell depletion of HLA-identical transplants in leukemia. Blood, 78(8), 2120–2130.
- Wade J. C. (2006). Viral infections in patients with hematological malignancies. Hematology. American Society of Hematology. Education Program, 368–374.
- Ćupić M. (2019). Herpesviridae. In: Savić B, Mitrović S, Jovanović T. (ured.) (2019). Medicinska mikrobiologija (497-507). Beograd: Medicinski fakultet Univerziteta u Beogradu, CIBID.
- Yuan, L., Li, S., Chen, Q., Xia, T., Luo, D., Li, L., Liu, S., Guo, S., Liu, L., Du, C., Jia, G., Li, X., Lu, Z., Yang, Z., Liu, H., Mai, H., & Tang, L. (2022). EBV infection-induced GPX4 promotes chemoresistance and tumor progression in nasopharyngeal carcinoma. Cell death and differentiation, 29(8), 1513–1527.
- Ljungman P. (2004). Viral infections: current diagnosis and treatment. The hematology journal : the official journal of the European Haematology Association, 5 Suppl 3, S63–S68.
- Sandherr, M., Einsele, H., Hebart, H., Kahl, C., Kern, W., Kiehl, M., Massenkeil, G., Penack, O., Schiel, X., Schuettrumpf, S., Ullmann, A. J., Cornely, O. A., & Infectious Diseases Working Party, German Society for Hematology and Oncology (2006). Antiviral prophylaxis in patients with haematological malignancies and solid tumours: Guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society for Hematology and Oncology (DGHO). Annals of oncology : official journal of the European Society for Medical Oncology, 17(7), 1051–1059.
- Busca A. (2012). Viral infections in patients with hematological malignancies. Leukemia supplements, 1(Suppl 2), S24–S25.
- Hallek, M., Cheson, B. D., Catovsky, D., Caligaris-Cappio, F., Dighiero, G., Döhner, H., Hillmen, P., Keating, M., Montserrat, E., Chiorazzi, N., Stilgenbauer, S., Rai, K. R., Byrd, J. C., Eichhorst, B., O’Brien, S., Robak, T., Seymour, J. F., & Kipps, T. J. (2018). iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood, 131(25), 2745–2760.
- Andrei, G., & Snoeck, R. (2021). Advances and Perspectives in the Management of Varicella-Zoster Virus Infections. Molecules (Basel, Switzerland), 26(4), 1132.
- Ma, S. D., Hegde, S., Young, K. H., Sullivan, R., Rajesh, D., Zhou, Y., Jankowska-Gan, E., Burlingham, W. J., Sun, X., Gulley, M. L., Tang, W., Gumperz, J. E., & Kenney, S. C. (2011). A new model of Epstein-Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas. Journal of virology, 85(1), 165–177.
- Ru, Y., Zhu, J., Song, T., Ding, Y., Zhu, Z., Fan, Y., Xu, Y., Sun, A., Qiu, H., Jin, Z., Tang, X., Han, Y., Fu, C., Chen, S., Ma, X., Chen, F., Chen, J., & Wu, D. (2022). Features of Epstein-Barr Virus and Cytomegalovirus Reactivation in Acute Leukemia Patients After Haplo-HCT With Myeloablative ATG-Containing Conditioning Regimen. Frontiers in cellular and infection microbiology, 12, 865170.
- Liu, T. Y., Wu, S. J., Huang, M. H., Lo, F. Y., Tsai, M. H., Tsai, C. H., Hsu, S. M., & Lin, C. W. (2010). EBV-positive Hodgkin lymphoma is associated with suppression of p21cip1/waf1 and a worse prognosis. Molecular cancer, 9, 32.
- Guan, H., Miao, H., Ma, N., Lu, W., & Luo, B. (2017). Correlations between Epstein-Barr virus and acute leukemia. Journal of medical virology, 89(8), 1453–1460.
- Ahmed, H. G., Osman, S. I., & Ashankyty, I. M. (2012). Incidence of Epstein-Barr virus in pediatric leukemia in the Sudan. Clinical lymphoma, myeloma & leukemia, 12(2), 127–131.
- Djuric, M., Jankovic, L., Jovanovic, T., Pavlica, D., Brkic, S., Knezevic, A., Markovic, D., & Milasin, J. (2009). Prevalence of oral herpes simplex virus reactivation in cancer patients: a comparison of different techniques of viral detection. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology, 38(2), 167–173.
- Yahav, D., Gafter-Gvili, A., Muchtar, E., Skalsky, K., Kariv, G., Yeshurun, M., Leibovici, L., & Paul, M. (2009). Antiviral prophylaxis in haematological patients: systematic review and meta-analysis. European journal of cancer (Oxford, England : 1990), 45(18), 3131–3148.
- Hong, J., Park, H. K., Park, S., Lee, A., Lee, Y. H., Shin, D. Y., Koh, Y., Choi, J. Y., Yoon, S. S., Choi, Y., & Kim, I. (2020). Strong association between herpes simplex virus-1 and chemotherapy-induced oral mucositis in patients with hematologic malignancies. The Korean journal of internal medicine, 35(5), 1188–1198.
- Park, L. C., Lee, H. S., Shin, S. H., Im, H., Ye, B. J., Song, M. K., Oh, S. Y., Lee, S. M., Lee, W. S., & Kim, Y. S. (2011). Herpesviridae viral infections following rituximab combined chemotherapy in patients with diffuse large B-cell lymphoma. Acta haematologica, 125(4), 230–236.
- Lee, H. S., Park, J. Y., Shin, S. H., Kim, S. B., Lee, J. S., Lee, A., Ye, B. J., & Kim, Y. S. (2012). Herpesviridae viral infections after chemotherapy without antiviral prophylaxis in patients with malignant lymphoma: incidence and risk factors. American journal of clinical oncology, 35(2), 146–150.
- Lorenzetti, M. A., Altcheh, J., Moroni, S., Moscatelli, G., Chabay, P. A., & Preciado, M. V. (2010). EBNA1 sequences in Argentinean pediatric acute and latent Epstein-Barr virus infection reflect circulation of novel South American variants. Journal of medical virology, 82(10), 1730–1738.
