1. Introduction
The human cytomegalovirus (CMV) is a pathogen with a worldwide distribution. The risk of contracting the virus is continuous and lifelong (Hecker et al. 2004), with a primary encounter occurring in most individuals during the first twenty years of life (Alshammari, 2017). As with all Herpesviridae, CMV establishes a lasting persistence within the host. More than half of the adults over the age of 40 have had previous contact with CMV (Centers for Disease Control and Prevention [CDC], 2020). This percentage increases up to 90-100% in some regions of the world where the virus is acquired in early childhood (Knox, 1983). The overall seroprevalence demonstrates great variability depending on a variety of circumstances. A plethora of factors contribute to CMV seropositivity: age (Ahlfors, 1984; Hecker et al. 2004; Staras et al. 2006), gender (Hecker et al. 2004; Bate et al. 2010; Varga et al. 2011), socioeconomic status (Marshall et al. 1993; Cannon et al. 2010; Lachmann et al. 2018), current smoking (Lachmann et al. 2018), level of education (Lachmann et al. 2018), number of sexual partners (Lachmann et al. 2018), childcare practices (Staras et al. 2006), different cultural conditions or customs related to breastfeeding (Staras et al. 2006), to name some of them.
A predominantly benign infectious agent in the healthy population, CMV is a notorious etiological driving force behind morbidity and mortality in patients with hematologic malignancies. CMV infection/reactivation may be considered among the most significant viral complications of allogeneic hematopoietic stem cell transplantation (HSCT) (Ljungman et al. 2004). Information on the prior exposure to this pathogen is important to obtain. Screening for the evidence of previous contact with CMV relies on serology. Determining donor and recipient serostatus belongs to precepts of transplantology, as it can influence the outcome of allogeneic HSCT (Ljungman and Brandan, 2007).
In the present study, we investigated CMV seroprevalence in 70 patients with lymphoid B-cell malignancies in relation to age, gender, and type of underlying blood cancer.
2. Materials and methods
2.1. Terminology
CMV seropositivity is defined by the presence of anti-CMV IgG antibodies in the serum, indicating a prior contact with the pathogen. CMV seroprevalence is classified as the prevalence of CMV seropositivity in a particular population (Cannon et al. 2010).
2.2. Study group
The subject cohort comprised 70 patients hospitalized in the Clinic of Hematology, University Clinical Center of Serbia, Belgrade, Republic of Serbia. All patients were of Caucasian descent. The males were slightly preponderant (33/37 = f/m; gender ratio, 0.89).
The mean age of the cohort was 52.3 years (range 21-73) with the females aged 48.1 years (range 21-73) and males aged 56 years (range 27-73). The age difference was not significant (p=0.073).
The patients were categorized on the grounds of age: 21-40 years (13 subjects, 18.6%), 41-60 years (36, 51.4%) and >60 years (21, 30%). Age categories were partitioned into 20- year clusters rather than 10- year groups, because of small frequencies that may have interfered with statistical inferences.
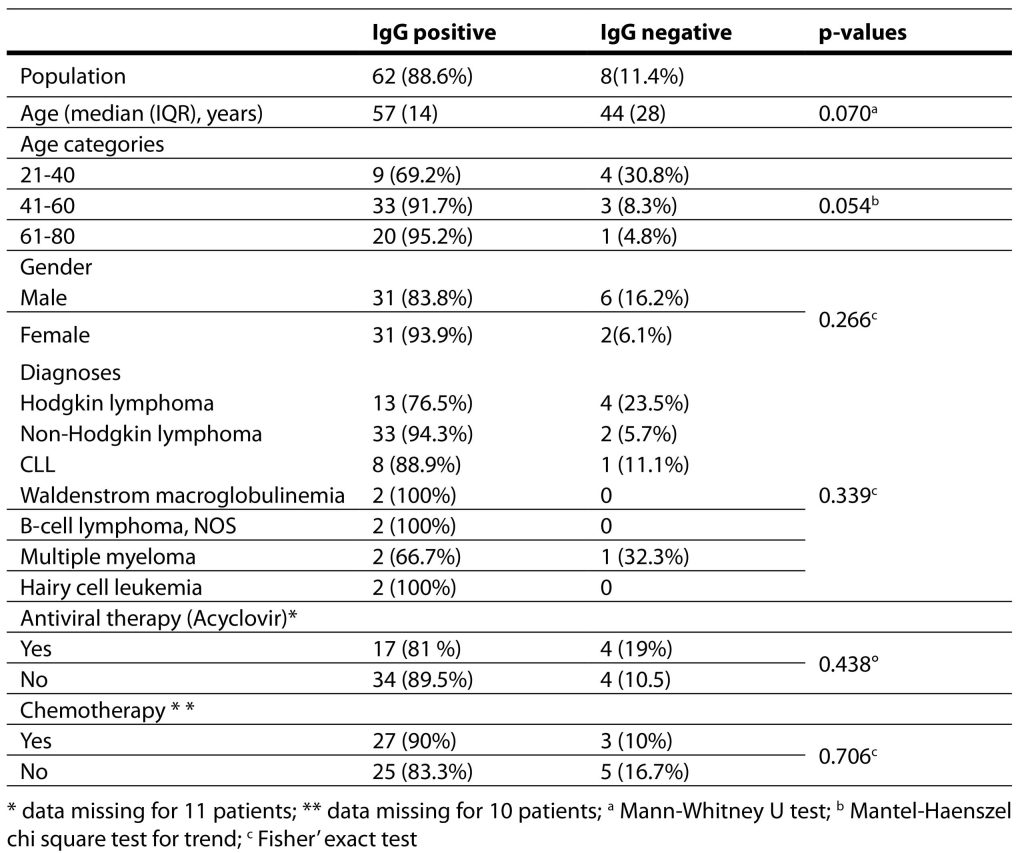
The patient characteristics are presented in Table 1.
Table 1. Patient characteristics with regards to seroprevalence.
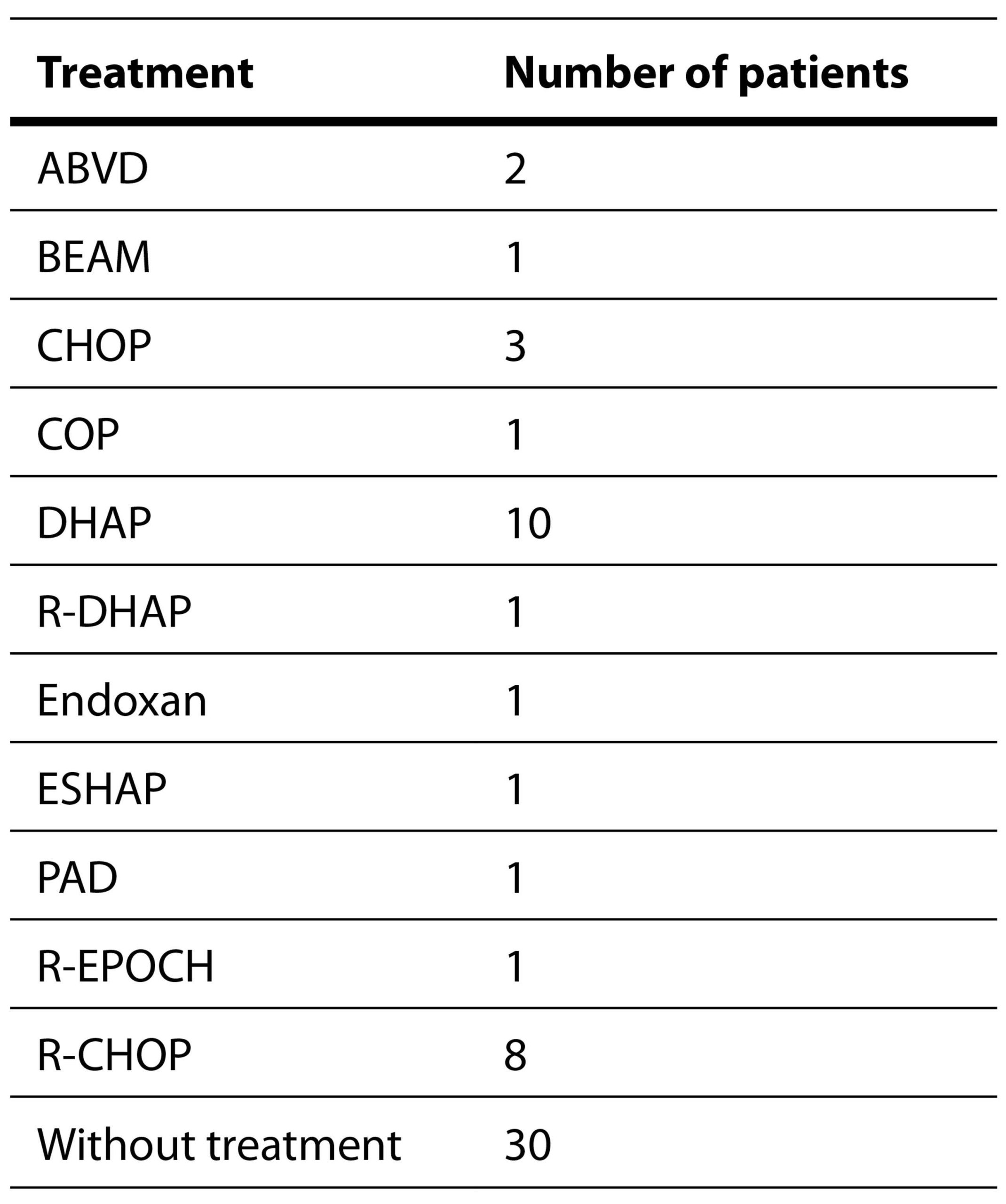
All patients were diagnosed with a lymphoid B-cell neoplasm. None of them were elected for HSCT. Thirty patients (42.8%) were on chemotherapeutic protocols at the time of sampling. The therapeutic regimens administered are showed in Table 2.
Table 2. Chemotherapy regimens used in the study.
ABVD – Doxorubicin hydrochloride (Adriamycin), Bleomycin sulfate, Vinblastine sulfate, and Dacarbazine
BEAM – Carmustine (BiCNU), Etoposide, Cytarabine (Ara-C, cytosine arabinoside), Melphalan
R-CHOP – Rituximab, Cyclophosphamide, Doxorubicin hydrochloride, Vincristine (Oncovin), Prednisolone
CTD – Cyclophosphamide, Thalidomide, Dexamethasone
DHAP – Dexamethasone, High-dose Ara-C, Platinol (cisplatin)
ESHAP – Etoposide, Methylprednisolone, High-dose Ara-C, Cisplatin
PAD – Bortezomib, Doxorubicin, Dexamethasone
R-CD – Rituximab, Cyclophosphamide, Dexamethasone
P-CVP – Rituximab, Cyclophosphamide, Vincristine, Prednisolone
R-EPOCH – Rituximab, Etoposide, Prednisone, Vincristine, Cyclophosphamide, Doxorubicin
Twenty-one patients (30%) received preventive doses of Acyclovir and 38 (54.3%) did not. Information regarding antivirals was missing in 11 (15.7%) patients. Antiviral prophylaxis for CMV infection was not routinely instituted.
2.3. Sample collection and analysis
Sampling was approved by the Ethics Committee of the Medical Faculty, University of Belgrade, with patients signing an informed consent. Blood samples were collected between February and November 2017 by venipuncture using standardized red-top vacutainers. Serologic analyses were performed at the Virology Laboratory of the Institute of Microbiology and Immunology, Faculty of Medicine, University of Belgrade. Antibody presence and classes were screened by using commercial anti-CMV ELISA IgM and IgG kits (Euroimmun, Lubeck, Germany), with antibody detection carried out via spectrophotometric analysis on an ELISA Reader 270 (bioMérieux, Paris, France).
2.4. Statistical analysis
Results are presented as count (percent) or median (interquartile range) depending on the data type. Group comparisons were performed using Fisher’s Exact test, Mann-Whitney U tests and Mantel-Haenszel Chi-square test for trend. All p-values <0.05 were considered significant. All data were analyzed using SPSS v. 20.0 statistical software (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.).
3. Results
The total CMV seroprevalence was 88.6% (62/70 patients). All patients were successfully tested for IgG antibodies. For technical reasons, conclusive evidence for the presence of IgM antibodies could not be obtained in 12 (17.1%) patients.
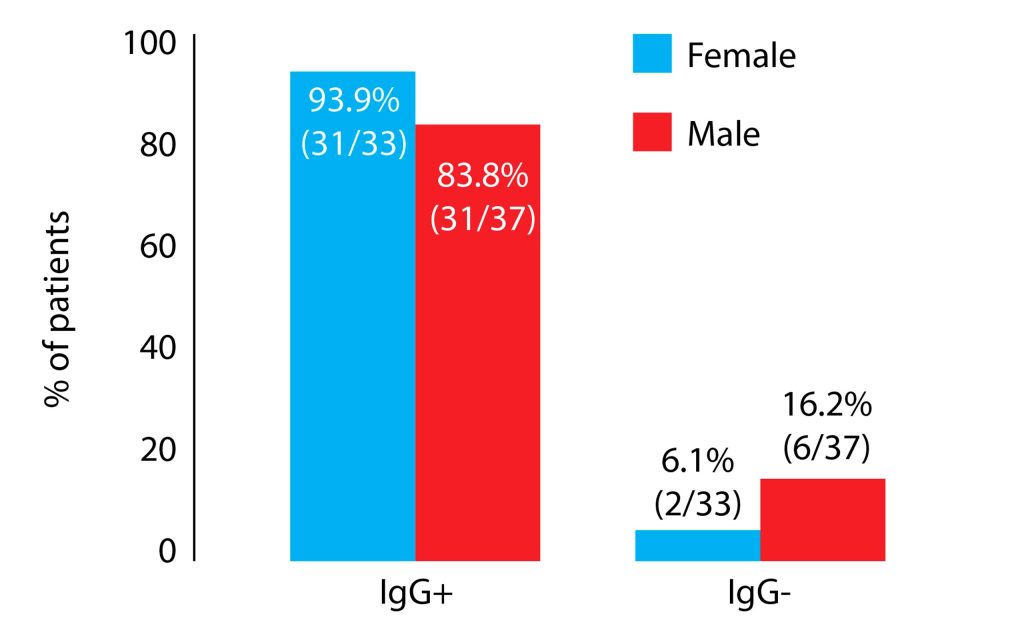
The IgG seropositivity was 83.8% in males and 93.9% in females. Although more females were IgG positive, statistical significance was not reached (p=0.266). All of the patients in whom the IgG antibodies were absent also tested negative for IgM. CMV seropositivity as regards gender is presented in Figure 1.
Figure 1. The prevalence of cytomegalovirus IgG antibody and gender.
Overall, 3 (5.2%) patients were IgM positive, while 5 (8.6%) were borderline positive (an equivocal result). The rest (50 patients, 86.2%) were IgM negative and information concerning IgM antibodies was not recovered in 12 patients.
In the male group only a single patient (3.2%) was IgM positive. Two (6.5%) samples gave an equivocal result. The rest of the males (90.3%) were IgM negative.
In female patients, 2 (7.4%) and 3 (11.1%) manifested IgM positive and equivocal results, respectively. The remaining 22 females (82.5%) were IgM negative. Women manifested more IgM positive events than men but the difference was not significant (p=0.620). The level of significance was not attained even if equivocal results were interpreted as positive (p=0.453).
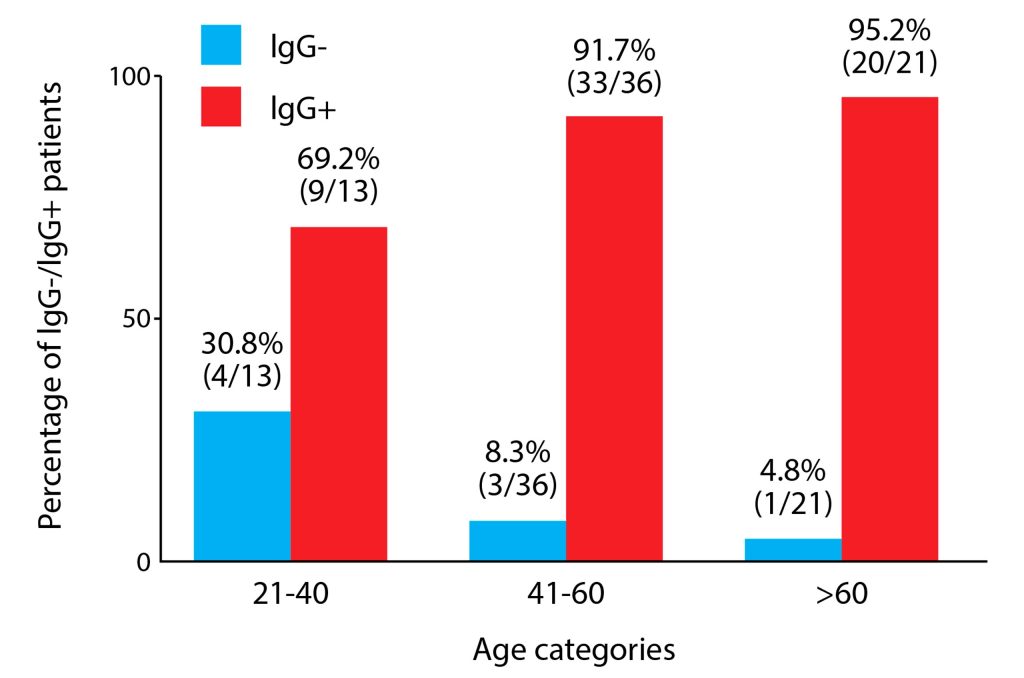
The CMV IgG positivity was higher in older participants than in the younger ones. This difference was close to significance (p=0.07).
Seroprevalence steadily increased from the first to the last age group, with the distribution across the age categories shown in Figure 2.
Figure 2. Distribution of cytomegalovirus seropositivity across distinct age groups.
Taking into account all age categories, there was no significant difference in CMV seropositivity between them; however, significance was almost achieved with p=0.054 (Mantel-Haenszel chi-square test for trend).
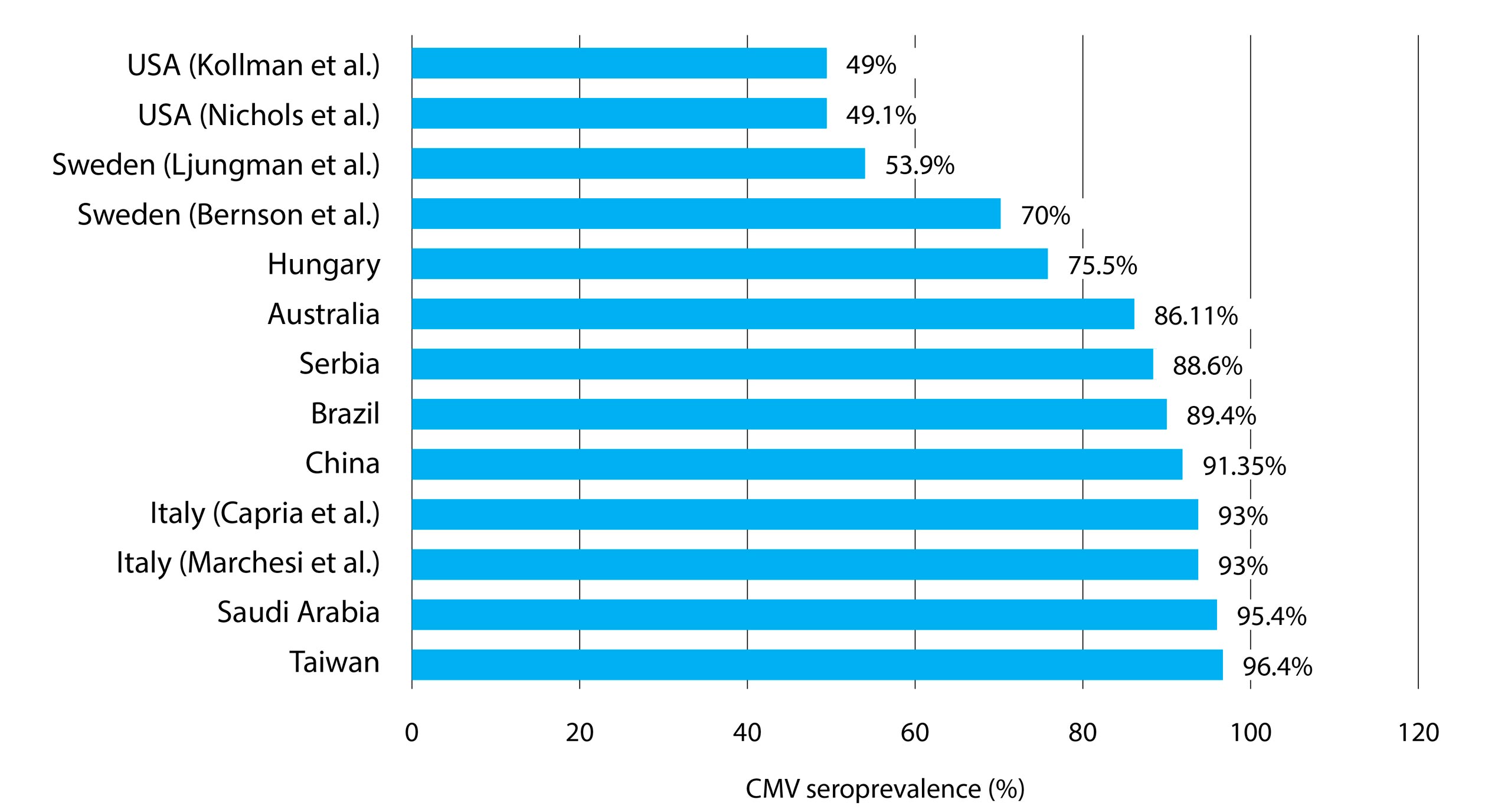
With regard to an underlying B-cell cancer, 2/2 subjects with hairy cell leukemia, 2/2 with Waldenström macroglobulinemia, and 2/2 with non-specified B-cell lymphoma (2/2) were IgG positive. However, there were too few patients. Patients with non-Hodgkin lymphoma (33/35, 94.3%) boasted most CMV IgG positives, followed by those with chronic lymphocytic leukemia (CLL) (8/9, 88.9%) and Hodgkin lymphoma (13/17, 76.5%). The lowest CMV seroprevalence was noted in patients diagnosed with multiple myeloma (2/3, 66.7%); again, the cohort was too small. Generally, CMV seropositivity did not correlate with the basic hematological neoplasm (p=0.339). The seroprevalences from other works referred to in this study are represented in Figure 3.
Figure 3. A vis-à-vis CMV seroprevalence by country referred to in the present research.
4. Discussion
To the best of our knowledge, this is the second study investigating CMV seroprevalence in adults with hematologic B-cell malignancies in the Republic of Serbia. The overall CMV seroprevalence in our study group was 88.6%. Similar results were reported in two demographically distinct populations, Brazil and Australia, with the respective seroprevalences of 89.4% (De Matos et al. 2011) and 86.11% (Ng et al. 2005). A possible bias should be noted, however, as from the latter study included only 36 patients. A comparable percentage of CMV positivity was reported by Xuan et al. (2012; Guangzhou, China), where 91.35% of subjects afflicted by various hematologic neoplasms were previously in contact with the virus. A study of multiple myeloma and lymphoma patients from Italy by Marchesi et al. (2015) observed a seroprevalence of 93%; an identical prevalence was noted in another Italian work involving patients with acute myeloid leukemia (Capria et al. 2010). Prior contact with CMV was verified in 95.4% of approximately 500 patients with diverse hematologic malignancies in Saudi Arabia (Abdul et al. 2019). An even greater occurrence was reported by Liu and coworkers (Taiwan) with 96.4% of recipients of the allogeneic HSCT being CMV IgG positive (Liu et al. 2012).
Reports on the CMV seroprevalence in blood cancer patients are similar to our population of B-cell neoplasms, although there are some exceptions. In a cohort of Swedish patients from a multicenter study (Bernson et al. 2018), only 70% of patients were positive for CMV IgG. A study from Hungary involving patients with lymphoid and myeloid hematologic malignancies and other hematologic diseases reported 75.5% of patients with CMV‒specific IgG antibodies (Piukovics et al. 2017). Significantly lesser numbers were noted by Ljungman and Brandan (Ljungman and Brandan, 2007) with 53.9% of hematological patients seropositive for CMV. This study covered more than 40,000 patients but their smaller prevalence may be explained by younger age of the examinees (mean age, 31 years), as it is known that CMV seropositivity tends to rise with age (Ahlfors, 1984; Hecker et al. 2004; Staras et al. 2006). Another factor contributing to a low prevalence may be a demographic one. Namely, their data included patients from socioeconomically developed European states (Netherlands, Belgium, France, Spain, Italy, United Kingdom, Germany, and Nordic countries). Hence, a younger age and better socioeconomic status (Marshall et al. 1993) may be an explanation for a lower prevalence of CMV contacts in the mentioned study (Ljungman and Brandan, 2007).
As there are several different routes of virus transmission, natural variations in CMV prevalence relative to gender and age are expected. Geographic location is a risk in itself as reflected by a differential prevalence between countries (Ahlfors, 1984; Hizel et al. 1999). Additionally, variations within a single country have been observed (Gratacap-Cavallier et al. 1998). A conspicuously lower CMV prevalence was reported by Kollman et al. (2001, USA) where only 49% of the graft recipients had a prior CMV infection. Similarly, research on consecutive HSCT recipients in USA yielded a seropositivity of 49.1% (Nichols et al. 2002). Considerably lesser seropositive proportions in these two studies are in line with a lower age-adjusted frequency of CMV infection in the USA (50.5%) (Bate et al. 2010).
Virus pervasiveness among the patient cohorts may have been influenced by differences in cohort sizes, age, gender ratio, and the methodology used for antibody detection. We did not account for the socio-economic background of the subjects, smoking habits, number of different sexual contacts, childcare practice, different cultural conditions or customs related to breastfeeding, etc. These may also have an impact on serostatus of the patients (Marshall et al. 1993; Staras et al. 2006; Cannon et al. 2010; Lachmann et al. 2018).
Females have a higher proportion of CMV seropositivity than males (Hecker et al. 2004; Bate et al. 2010; Varga et al. 2011). Our study is in support of these findings, albeit significance was not attained in our population (p=0.266). In a study on HSCT recipients by Ljungman and Brandan (2007) female patients were significantly more likely to be CMV seropositive. A higher risk for the female sex to develop CMV seropositivity was reported by Staras et al. (2006). Similarly, a preponderance (not significant) of CMV seropositive females with B-CLL was reported by Pourgheysari et al. (2010). In a group of Brazilian patients with various hematologic disorders females were CMV seropositive more frequently than males, but not significantly (De Matos et al. 2011). Conversely, in a group of leukemia patients from Sudan, females were infected with CMV less often (Dafalla et al. 2018).
Age is apparently a significant demographic risk factor for CMV seropositivity. The two show a tendency to increase in parallel in study populations (Ahlfors, 1984; Hecker et al. 2004; Staras et al. 2006; Seale et al. 2006). In a report on hematologic patients, the risk of encountering CMV increases with age in females and males alike (Ljungman and Brandan, 2007). Contrariwise, age was not found to be a significant predictor of CMV prevalence in Khartoum State, Sudan (Dafalla et al. 2018). In another cohort of patients with B-cell CLL, there was no difference in CMV seronegative vs. CMV seropositive groups of patients with respect to age (Pourgheysari et al. 2010). Patients with diverse hematological diseases were investigated elsewhere (De Matos et al. 2011) employing age stratification similar to one presented in our study. These reports describe the highest CMV prevalence in patients over 58 years (De Matos et al. 2011) in Bahia State (Brazil) with a clear-cut statistical significance. Significant difference in IgG positivity between age groups was not attained in the current study (p=0.054). A larger patient cohort may have compensated for these nuances yielding decisive conclusions.
Methods employed in detection of CMV IgG and IgM antibodies vary in sensitivity. Discrepancies in determining CMV seroprevalence between our work and other studies may stem from various methodologies employed since chemiluminescence, indirect immunofluorescence, and ELISA were all used in different studies herein referred to.
In the present study, only two patients with hairy cell leukemia, Waldenström macroglobulinemia, and non-specified B-cell lymphoma each were respectively 100% CMV seropositive. We discounted these patient groups because of a too small number of subjects. The largest bodies of participants in our study, those with non-Hodgkin lymphoma and CLL, manifested the highest seroprevalence. A study by Abdul et al. (2012) demonstrated the largest prevalence of CMV in patients with CLL, multiple myeloma, and myelodysplastic syndromes which partially corroborates our findings. Patients with myeloproliferative neoplasms had a lower prevalence of CMV infection (87.5%) in this study. These authors have not demonstrated a significant difference in seroprevalence across different underlying diseases, which accords with our results.
The limitation of the current study is a small number of patients per underlying neoplasm. More precise data could have been obtained had the population been larger. Also, the socio-economic aspect of our participants was not considered – an important link to CMV infection. Nonetheless, presumably the seroprevalence would be higher if a larger number of patients were studied – namely, the Republic of Serbia belongs to developing countries which supports an expectation of a high incidence of CMV contacts (Chakravarty et al. 2005). There is a geographical gradient across Europe with a higher seroprevalence in southern than in the northern parts of the continent. The Nordic countries form an exception to this trend with prevalence similar to that in Southern Europe (Ljungman and Brandan, 2007). This begs a categorical interpretation. Day care centers were introduced earlier in Nordic countries than in the rest of Europe which may be one plausible explanation. Although our study included only Caucasians, this does not make our region a biased one because the Serbian population is bona fide consisting only of whites. Finally, administration of blood products is not infrequently performed in hematological patients even before the treatment commences. Therefore, a passive transfer of donor CMV IgG antibodies cannot be entirely excluded. For our subjects, the full extent of blood and blood product transfusion prior to chemotherapy was not known. In consequence, this data was not used.
5. Conclusion
The evidence presented favors a tendency of CMV seropositivity to prevail in females rather than males which accords with similar studies. Older patients are more frequently CMV IgG positive than the younger. Our results do not demonstrate an unambiguous difference between discrete age categories and the CMV seroprevalence – a larger patient cohort might accomplish stronger statistical inferences. No one underlying B-cell malignancy had an advantage in CMV prevalence over another. Involvement of more participants, adjusting populations for gender and age, as well as detailed investigation of other factors influencing CMV seropositivity might allow for more precise results.
Acknowledgements: We owe our gratitude to the Ministry of Education, Science and Technological Development for supporting this research via MPNTR grant, project no. 451-03-66/2024-03/200110. The authors are also grateful to the patients and their families, and appreciate the contribution of associated clinical support services and staff in the completion of this study.
Funding: This research was supported by the MPNTR grant of the Ministry of Education, Science and Technological Development, project no. 451-03-66/2024-03/200110. The Ministry as the funding source had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Conflicts of interest/Competing interests: The authors declare that they have no conflict of interest.
Ethics approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the University of Belgrade, Faculty of Medicine, Belgrade, Republic of Serbia.
References
Abdul, Rehman, Z., Zaidi, Maged, O., Al-Ammari, Mohammed, AlNoamani, Syed, Y., Altaf, Nawal, AlShehry, Imran, K., Tailor, Ibraheem, H., Motabi, Mubarak, S., AlGhamdi, & Syed, Ziauddin, A., Zaidi. (2019). Very High Seroprevalence of CMV and EBV Among a Large Series of Patients with Hematological Malignancies at a Tertiary Care Center in Saudi Arabia – a Case for Investigating Cooperativity of Viruses in Carcinogenesis?. Blood, 134 (Supplement_1), 5818.
Ahlfors K. (1984). IgG antibodies to cytomegalovirus in a normal urban Swedish population. Scandinavian journal of infectious diseases, 16(4), 335–337.
Alshammari, F., D. (2017). Association between HPV, CMV, EBV and HS Viruses and Breast Cancer in Saudi Arabia. J Cancer Prev Curr Res, 7(3), 74-79.
Bate, S. L., Dollard, S. C., & Cannon, M. J. (2010). Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America, 50(11), 1439–1447.
Bernson, E., Hallner, A., Sander, F. E., Nicklasson, M., Nilsson, M. S., Christenson, K., Aydin, E., Liljeqvist, J. Å., Brune, M., Foà, R., Aurelius, J., Martner, A., Hellstrand, K., & Thorén, F. B. (2018). Cytomegalovirus Serostatus Affects Autoreactive NK Cells and Outcomes of IL2-Based Immunotherapy in Acute Myeloid Leukemia. Cancer immunology research, 6(9), 1110–1119.
Cannon, M. J., Schmid, D. S., & Hyde, T. B. (2010). Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Reviews in medical virology, 20(4), 202–213.
Capria, S., Gentile, G., Capobianchi, A., Cardarelli, L., Gianfelici, V., Trisolini, S. M., Foà, R., Martino, P., & Meloni, G. (2010). Prospective cytomegalovirus monitoring during first-line chemotherapy in patients with acute myeloid leukemia. Journal of medical virology, 82(7), 1201–1207.
Centers for Disease Control and Prevention. (2020). Cytomegalovirus (CMV) and Congenital CMV infection.
Chakravarty, A., Kashyap, B., & Rathi, K. (2005). The seroepidemiological study on cytomegalovirus in women of child-bearing age with special reference to pregnancy and maternal-fetal transmission. Indian journal of pathology & microbiology, 48(4), 518–521.
Dafalla, A., B., Y., Elnil, Y., F., H., & Gorish, B., M., T. (2018). Seroprevalence of Cytomegalovirus Infection among Leukemic Patients in Khartoum State. Virol Mycol, 7, 2-7.
De Matos, S. B., Meyer, R., & Lima, F. W. (2011). Seroprevalence and serum profile of cytomegalovirus infection among patients with hematologic disorders in Bahia State, Brazil. Journal of medical virology, 83(2), 298–304.
Gratacap-Cavallier, B., Bosson, J. L., Morand, P., Dutertre, N., Chanzy, B., Jouk, P. S., Vandekerckhove, C., Cart-Lamy, P., & Seigneurin, J. M. (1998). Cytomegalovirus seroprevalence in French pregnant women: parity and place of birth as major predictive factors. European journal of epidemiology, 14(2), 147–152.
Hecker, M., Qiu, D., Marquardt, K., Bein, G., & Hackstein, H. (2004). Continuous cytomegalovirus seroconversion in a large group of healthy blood donors. Vox sanguinis, 86(1), 41–44.
Hizel, S., Parker, S., & Onde, U. (1999). Seroprevalence of cytomegalovirus infection among children and females in Ankara, Turkey, 1995. Pediatrics international: official journal of the Japan Pediatric Society, 41(5), 506–509.
Knox G. E. (1983). Cytomegalovirus: import of sexual transmission. Clinical obstetrics and gynecology, 26(1), 173–177.
Kollman, C., Howe, C. W., Anasetti, C., Antin, J. H., Davies, S. M., Filipovich, A. H., Hegland, J., Kamani, N., Kernan, N. A., King, R., Ratanatharathorn, V., Weisdorf, D., & Confer, D. L. (2001). Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood, 98(7), 2043–2051.
Lachmann, R., Loenenbach, A., Waterboer, T., Brenner, N., Pawlita, M., Michel, A., Thamm, M., Poethko-Müller, C., Wichmann, O., & Wiese-Posselt, M. (2018). Cytomegalovirus (CMV) seroprevalence in the adult population of Germany. PloS one, 13(7), e0200267.
Liu, Y. C., Lu, P. L., Hsiao, H. H., Chang, C. S., Liu, T. C., Yang, W. C., & Lin, S. F. (2012). Cytomegalovirus infection and disease after allogeneic hematopoietic stem cell transplantation: experience in a center with a high seroprevalence of both CMV and hepatitis B virus. Annals of hematology, 91(4), 587–595.
Ljungman, P., & Brandan, R. (2007). Factors influencing cytomegalovirus seropositivity in stem cell transplant patients and donors. Haematologica, 92(8), 1139–1142.
Ljungman, P., Reusser, P., de la Camara, R., Einsele, H., Engelhard, D., Ribaud, P., Ward, K., & European Group for Blood and Marrow Transplantation (2004). Management of CMV infections: recommendations from the infectious diseases working party of the EBMT. Bone marrow transplantation, 33(11), 1075–1081.
Marchesi, F., Pimpinelli, F., Gumenyuk, S., Renzi, D., Palombi, F., Pisani, F., Romano, A., Spadea, A., Papa, E., Canfora, M., Ensoli, F., & Mengarelli, A. (2015). Cytomegalovirus reactivation after autologous stem cell transplantation in myeloma and lymphoma patients: A single-center study. World journal of transplantation, 5(3), 129–136.
Marshall, G. S., Rabalais, G. P., Stewart, J. A., & Dobbins, J. G. (1993). Cytomegalovirus seroprevalence in women bearing children in Jefferson County, Kentucky. The American journal of the medical sciences, 305(5), 292–296.
Ng, A. P., Worth, L., Chen, L., Seymour, J. F., Prince, H. M., Slavin, M., & Thursky, K. (2005). Cytomegalovirus DNAemia and disease: incidence, natural history and management in settings other than allogeneic stem cell transplantation. Haematologica, 90(12), 1672–1679.
Nichols, W. G., Corey, L., Gooley, T., Davis, C., & Boeckh, M. (2002). High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. The Journal of infectious diseases, 185(3), 273–282.
Piukovics, K., Terhes, G., Gurbity-Pálfi, T., Bereczki, Á., Rárosi, F., Deák, J., Borbényi, Z., & Urbán, E. (2017). Cytomegalovirus infection in patients with haematological diseases and after autologous stem cell transplantation as consolidation: a single-centre study. Annals of hematology, 96(1), 125–131.
Pourgheysari, B., Bruton, R., Parry, H., Billingham, L., Fegan, C., Murray, J., & Moss, P. (2010). The number of cytomegalovirus-specific CD4+ T cells is markedly expanded in patients with B-cell chronic lymphocytic leukemia and determines the total CD4+ T-cell repertoire. Blood, 116(16), 2968–2974.
Seale, H., MacIntyre, C. R., Gidding, H. F., Backhouse, J. L., Dwyer, D. E., & Gilbert, L. (2006). National serosurvey of cytomegalovirus in Australia. Clinical and vaccine immunology: CVI, 13(11), 1181–1184.
Staras, S. A., Dollard, S. C., Radford, K. W., Flanders, W. D., Pass, R. F., & Cannon, M. J. (2006). Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America, 43(9), 1143–1151.
Varga, M., Görög, D., Kári, D., Környei, E., Kis, É., Túryné, H. J., Jankovics, I., Péter, A., Toronyi, É., Sárváry, E., Fazakas, J., & Reusz, G. (2011). Cytomegalovirus seroprevalence among solid organ donors in Hungary: correlations with age, gender, and blood group. Transplantation proceedings, 43(4), 1233–1235.
Xuan, L., Huang, F., Fan, Z., Zhou, H., Zhang, X., Yu, G., Zhang, Y., Liu, C., Sun, J., & Liu, Q. (2012). Effects of intensified conditioning on Epstein-Barr virus and cytomegalovirus infections in allogeneic hematopoietic stem cell transplantation for hematological malignancies. Journal of hematology & oncology, 5, 46.