1. Introduction
Bacteria belonging to the genus Salmonella are facultatively Gram-negative rod-shaped motile bacteria and one of the most common zoonotic intestinal pathogens. Human and farm animal digestive tracts are bacterial primary niches. Salmonella enterica, as the human primary pathogen, has a very complicated serological classification, based on somatic, flagelar, and capsular antigens. Up to today, there are over 2600 serovars. Bacteria are divided into typhoidal and nontyphoidal serovars. Typhoidal serovars (S. Typhi and S. Paratyphi) are human-restricted, causing typhoid fever and leading to an estimated 11–21 million cases and 148,000–161,000 associated deaths every year (Hancuh et al. 2022). Nontyphoidal serovars are diverse in their host range and vary in their pathogenic capability. So they cause huge economic losses due to human and animal infections. Among humans, the most prevalent NTS causing enteric fevers are S. Enteritidis and S. Typhimurium. Only in the USA, contaminated chicken, turkey, or eggs have been linked to over 70% of cases of human salmonellosis (Andino & Hanning, 2015).
Biofilms are essential to the survival of microorganisms in their natural habitat, either the environment or the human body. Biofilm represents a sessile bacterial community and enables bacteria to survive in the harsh environment. Inside the biofilm, bacteria produce a protective matrix layer that is absent in planktonic lifestyle and is responsible for the majority of mechanisms that bacteria avoid eradication from the infection site. Bacterial communities inside biofilms can increase resistance to the host immune system. Thus, it is believed that over 80% of chronic infections are caused by biofilm-producing bacteria, which increases the risk of hospitalization, healthcare expenses, and morbidity (Vestby et al. 2020). Biofilms play a significant role in the chronic gallbladder carriage of S. Typhi, and are often associated with the presence of gallstones or other gallbladder abnormalities enabling S. Typhi an appropriate surface to establish biofilms (Harell et al. 2021). Biofilm formation is usually a bacterial response to changes in the environment or human organism and can be triggered by changes in pH, oxygen level, nutrient availability, or temperature in the habitat niche.
This study aimed to investigate the ability of nontyphoidal Salmonella strains isolated from contaminated food of animal origin to produce biofilm in different incubation conditions such as nutrient media and incubation temperatures.
2. Material and methods
2.1. Bacterial isolates
Bacterial strains analyzed in this study were part of the bacterial collection of the Center for Laboratory Testing and Quality in the Public Institution Veterinary Institute of the Republic of Srpska “Dr. Vaso Butozan”. Bacterial isolates were recovered from the food samples of the animal origin. A total of 730 samples were collected between 2017 and 2018 from different commercial markets. For pre-enriching the food samples, 25 g of the sample and 225 mL of 2% buffered peptone water (Oxoid, Thermo Scientific, Lenexa, KS) were placed in a bag and homogenized for 2 min in stomacher at the maximum speed (Bag Mixer; Interscience, Saint Nom, France). The homogenized food samples were then incubated at 37°C for 24 h. Next, 100 μL of each enriched sample was pipetted into 10 mL of Rappaport-Vassiliadis broth (Oxoid, Thermo Scientific, Waltham, MA) and incubated at 42°C for 24 h. Aliquots of the incubated Rappaport-Vassiliadis broth were streaked on xylose−lysine−deoxycholate agar (XLD, Oxoid, Thermo Scientific, Waltham, MA) using a loop, and each plate was incubated at 37°C for 24 h. Presumptive pink colonies were cultured and biochemically tested including Triple Sugar Iron (TSI), Citrate utilization, Urease, Sulphate, Indole, and motility tests. The Salmonella-positive colonies were serotyped by slide agglutination using commercially available antisera kits (Novolab, Belgium) with O and H antigen sera and interpreted in accordance to the Kaufmann-White scheme.
2.2. Biofilm production assay
Quantification of biofilm production was done following the protocol by Stepanovic et al (2004). After the 37°C overnight incubation in Trypticase Soy broth (TSB, Biorad, UK) and Brainheart Infusion broth (BHIB, Biorad, UK), strains were diluted in fresh media to achieve a final concentration of 106 CFU/ml. Aliquots of Salmonella spp. suspensions (100 μl) were transported to each well of the 96-well microtiter plate and afterward incubated for 24 hours at three different temperatures (4 °C, 25 °C, and 37 °C). Microtiter plates were aspirated and washed three times with sterile phosphate-buffered saline (PBS). The plates were dyed with 100 μl of 2% (w/v) crystal violet for 15 minutes, washed three times, and dried overnight at room temperature. Thereafter, 100 μl of glacial acetic acid at 33% (v/v) was used to dissolve the dye that had been bound to the biofilm matrix. Using an automated microtiter plate reader, the optical density (OD) of each well was determined at 570 nm (ICN Flow Titertek Multiskan Plus Reader, Meckenheim, Germany). TSB/BHIB broth was the only suspension in the negative control wells. Three standard deviations more than the mean OD of the negative control were designated as the cut-off OD (ODc).
The results were evaluated as follows:
OD ≤ ODc non-biofilm producers,
ODc < OD ≤ (2 × ODc) = weak biofilm producers,
(2 × ODc) < OD ≤ (4 × ODc) = moderate biofilm producers, and
OD > (4 × ODc) = strong biofilm producers.
Biofilm biomass produced by bacterial isolates was measured by using a spectrophotometer and expressed as the average OD for each isolate.
2.3. Statistical analysis
SPSS version 20.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Fisher’s exact test was used for comparison of the frequency of occurrence of the analyzed categorical variables. A p-value less than 0.05 was considered to be significant.
3. Results
3.1. Bacterial isolates
During two years, 30 isolates were detected in a total quantity of 730 samples (4.1%). The majority of isolates were recovered from chicken meat (27), while three strains were isolated from milk products and ground beef. The S. Infantis (14 isolates) and S. Enteritidis (12) were the two most common serotypes. We also detected two isolates of S. Typhimurium and one isolate of S. Virchow and S. Hadar.
3.2. Biofilm production
The bacterial capacity to produce biofilm was determined in different cultivation conditions. First, we wanted to study the formation of biofilm at different incubation temperatures in the provided broths. Next, we aimed to test whether two broths with different compositions are equally effective for biofilm production.
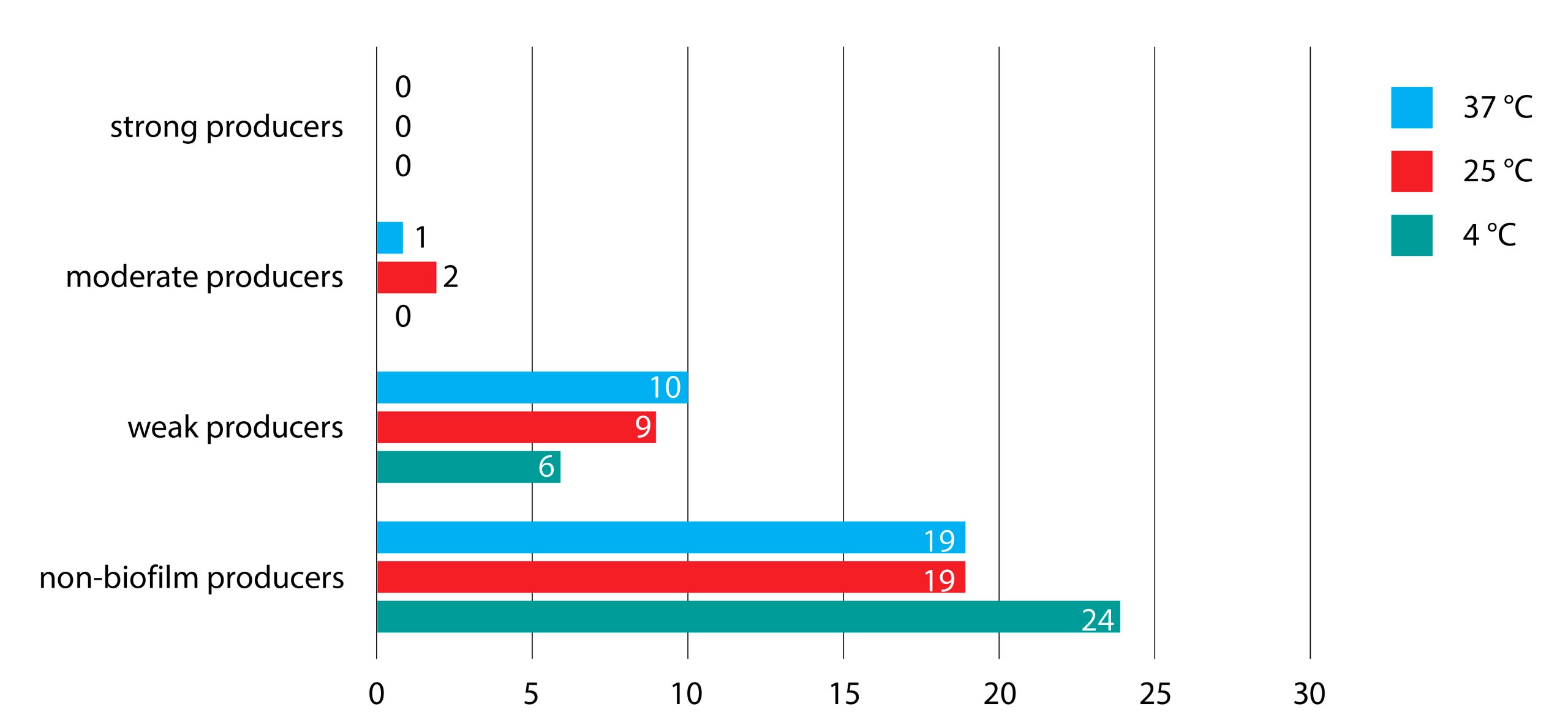
As expected, none of the isolates were strong biofilm producers at all three incubation temperatures in BHI broth. Although the refrigeration temperature was the most unfavorable for biofilm creation, there were no significant statistical differences between tested incubation temperatures (p=0.43), as shown in Figure 1.
Figure 1. Biofilm production of Salmonella serotypes in BHI broth in three different incubation temperatures
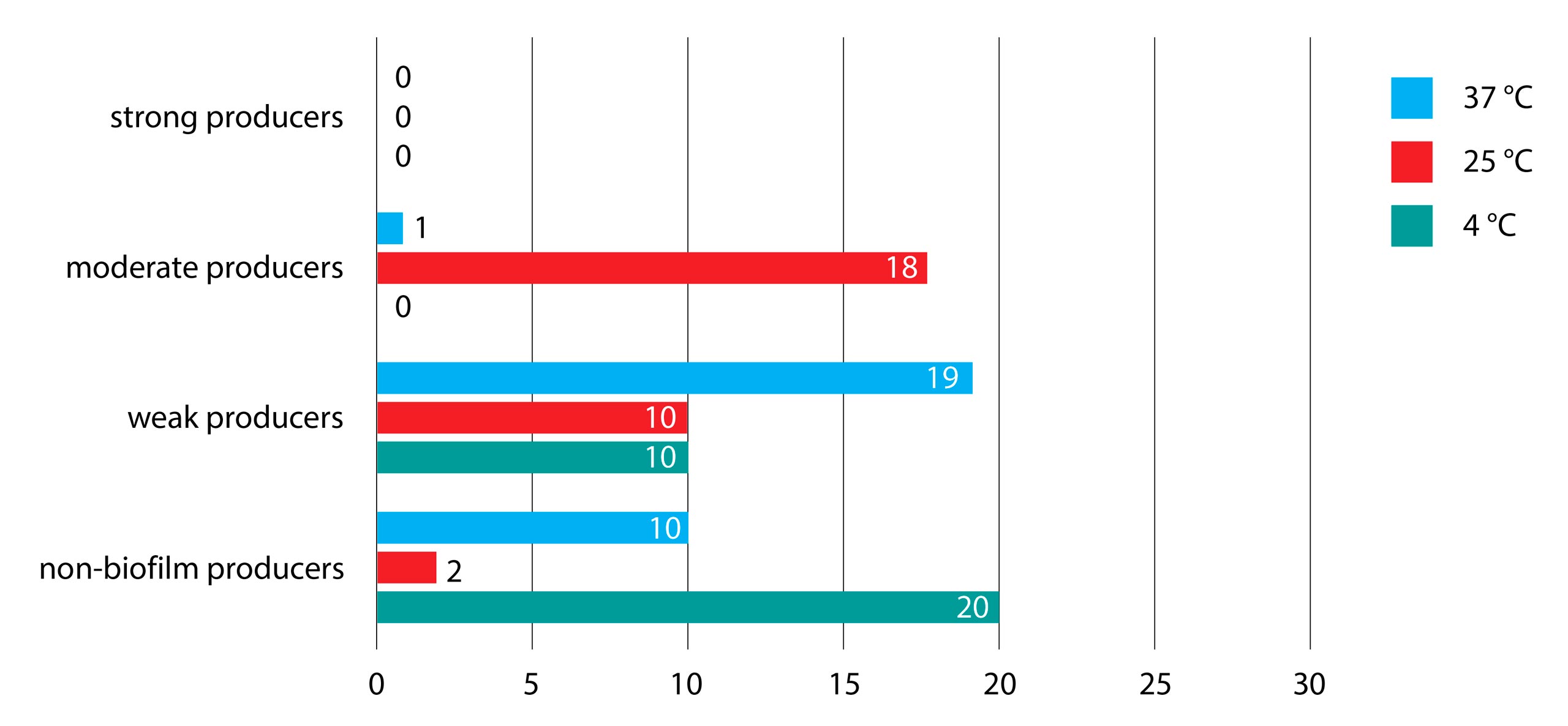
Also, in TSB broth we did not notice any strong biofilm producers in all three tested temperatures. On the other hand, at 4 °C we detected the majority of non-biofilm producers, while 25 °C was the most favorable temperature for moderate biofilm production. Weak biofilm production was detected mostly in isolates incubated at ambient temperature. Significant statistical difference was found between isolates incubated at all three temperatures (p<0.001, p<0.001, p<0.001). The lowest temperature was the least suitable for the formation of biofilm, whereas the sessile community thrived at ambient temperature, as shown in Figure 2.
Figure 2. Biofilm production of Salmonella serotypes in TSB broth at three different incubation temperatures
When we compared the biofilm production of different bacterial isolates in two different broths, we observed that TSB was the appropriate broth for better biofilm production during overnight incubation at 25 °C and 37 °C (p <0.001, p=0.049). This was because isolates cultivated in TSB showed more measured biofilm biomass (0.065 vs 0.032, and 0.029 vs 0.025, respectively) and a higher percentage of moderate and weak biofilm producers than in BHI broth. During overnight incubation at 4 °C, we noticed that biofilm biomass was evenly created in both broths (0.023 vs 0.022), as well as only a small percentage of weak biofilm producers were detected (33% vs 20%) (p=0.24).
4. Discussion
Worldwide, nontyphoidal salmonellosis is a serious public health concern in both industrialized and developing countries and is causing 93.8 million cases of gastroenteritis globally each year, with 155,000 deaths (Ngogo et al., 2020). The most common sources of human infections are poultry, eggs, and pork meat products, while herbal products (rice, fresh vegetables and fruits, seeds, and spices are the rarest types of products (Pinedo et al. 2022). This is in correlation with our findings of bacterial presence mostly in contaminated chicken meat. Salmonella can be transmitted in poultry horizontally through the contaminated feed or vertically from chicken to the egg. Bacteria can persist for long periods on farms among broilers and on non-living equipment through biofilm production and antibiotic resistance. The dominant serotypes in our study were S. Infantis and S. Enteritidis, with a common incidence of 87% of all tested isolates. Similar results were obtained from a previous survey of food samples of animal origin in our region, i.e. in Serbia and Croatia (Vidaković et al. 2017, Jaki Tkalec et al. 2021). According to reports from the European Food Safety Authority and the European Center for Disease Control and Prevention (2021), the two most common serotypes found in our study were also the most frequently isolated serotypes in broiler chickens. Broiler chicken meat was the most common sample in our research. Our results are also in agreement with the most prevalent serotypes detected among patients with bacterial gastroenteritis in Bosnia and Herzegovina (Aljicevic et al. 2019, Zdravstveno stanje stanovništva Republike Srpske 2021). S. Enteritidis and S. Typhimurium are invasive serotypes and cause erosion of epithelial cells and reduction of the intestinal surface area in broilers and subsequent transition into blood, bacteremia, and death. On the other hand, S. Infantis is not so invasive and lethal for broilers but can change the architecture of the intestinal mucosa (villus-height-to-crypt-depth ratios in the duodenum, jejunum, and ileum) leading to deterioration of feed degradation and absorption (Šefcova et al. 2023). Compared to slow-growing lines and layer-flocks, S. Infantis has a higher prevalence in fast-growing broilers, possibly due to lower immune response in the chicken gut and higher fecal shedding. (Drauch et al, 2022). The actuality of S. Infantis is explained by its efficient and rapid spread in poultry farms, with the consequent increase in incidence among human isolates. This serotype exhibits significant antibiotic resistance and, when combined with its ability to produce biofilm, allows long-term bacterial persistence in poultry farms.
Besides the farms, bacterial biofilms are also problematic in medicine, the food industry, water supply, and sewerage, as well as during the preparation of catering and household food. When outside of a host, Salmonella can form a biofilm, which allows it to attach to various surfaces, both living and non-living, and remain in a dormant but viable state until it’s ingested by a human or animal. In this study, we examined the capacity of NTS to create a biofilm under various cultivation conditions such as highly and moderately nutritious broths and at different incubation temperatures, which correspond to the temperature of the refrigerator, the ambient temperature, and the temperature of the human body. Our results showed that the composition of the broth has a huge impact on the bacterial capacity for biofilm production. Biofilm formation is a common adaptation mechanism used by bacteria to survive harsh environmental conditions, such as low humidity or lack of nutrients. After conducting our investigation, it became apparent that utilizing a highly nutritious medium such as BHI broth was not a suitable medium for testing biofilm production in our strains. This is because the majority of isolates we tested did not produce biofilm at all, and the remaining isolates that were capable of biofilm formation were classified as weak biofilm producers. Although it seems that the refrigeration temperature was the least favorable for biofilm formation, all isolates produced equal biofilm at all three tested temperatures in the BHI medium. On the other hand, a moderately nutritious medium such as TSB, was more suitable for biofilm testing since the majority of isolates were determined as low or moderate biofilm producers at higher temperatures (25 °C and 37 °C). Our findings are in agreement with the results of Stepanovic et al. (2004), who also observed that a highly nutritious medium like BHI is not suitable for testing the production of biofilms in Salmonella isolates. According to Speranza et al. (2011), a peptone concentration of 1.0-1.5 g/L is optimal for Salmonella spp. biofilm formation, which is more similar to peptone concentration in TSB than BHI broth used in this study. A significant statistical difference was observed in the ability of isolates to produce biofilm at various temperatures in the TSB medium. The study revealed that the lowest temperature was not a suitable condition for biofilm production, while the ambient temperature was the most favorable for the production of biofilm. When comparing the capacity of bacterial isolates for biofilm production at the same temperature between TSB and BHI broths, they showed better biofilm formation at higher temperatures in TSB compared to BHI. The most favorable temperature for biofilm formation in TSB was ambient temperature, which is in agreement with the results of other investigators (Speranza et al. 2011, Piras et al. 2015, Shatila et al. 2021). This could be explained by two different mechanisms of action. While 25 °C represents the ambient temperature and favors the optimal expression of agfD promoter, 37 °C is the optimal growth temperature of the pathogen. Goveart et al. (2018) explained poor biofilm formation at 37 °C due to a low expression of the agfD promoter at 37 °C and in a medium with high osmolarity. agfD promoter is important for the optimal synthesis of curli fimbriae and cellulose production which are important components of the biofilm matrix in Salmonella serotypes (Borges et al. 2018). At higher incubation temperatures (37 °C), which is the optimal temperature for bacterial growth and multiplication, weaker biofilm production can be observed. This can be attributed to the fast depletion of nutrients, the lack of curli biosynthesis, and the decrease in the viscosity of exopolysaccharides. (Shatila et al. 2021)
No difference between these two broths was noticed only at the refrigeration temperature. However, it is important to note that even at the lowest temperature, a small percentage of isolates managed to form a weak biofilm. Webber et al. (2019) showed that S. Enteritidis can form biofilm at low temperatures and short contact times, which is important because of difficulties in the disinfection of food industry surfaces and the possibility of cross-contamination during food processing.
5. Conclusion
Incubation conditions such as temperature and medium compositions are very important factors for biofilm production. Salmonella’s ability for biofilm formation at ambient and refrigeration temperatures is very important in the poultry industry, catering, and household and enables bacterial persistence for a long time.
Funding: This research received no external funding.
Informed Consent Statement: Not applicable.
Data Availability Statement: All data are presented in the article.
Conflicts of Interest: The authors declare no conflicts of interest.
References:
Hancuh, M., Walldorf, J., Minta, A. A., Tevi-Benissan, C., Christian, K. A., Nedelec, Y., Heitzinger, K., Mikoleit, M., Tiffany, A., Bentsi-Enchill, A. D., & Breakwell, L. (2023). Typhoid Fever Surveillance, Incidence Estimates, and Progress Toward Typhoid Conjugate Vaccine Introduction – Worldwide, 2018-2022. MMWR. Morbidity and mortality weekly report, 72(7), 171–176. https://doi.org/10.15585/mmwr.mm7207a2
Andino, A., & Hanning, I. (2015). Salmonella enterica: survival, colonization, and virulence differences among serovars. The Scientific World Journal, 2015, 520179. https://doi.org/10.1155/2015/520179
Vestby, L. K., Grønseth, T., Simm, R., & Nesse, L. L. (2020). Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics (Basel, Switzerland), 9(2), 59. https://doi.org/10.3390/antibiotics9020059
Harrell, J. E., Hahn, M. M., D’Souza, S. J., Vasicek, E. M., Sandala, J. L., Gunn, J. S., & McLachlan, J. B. (2021). Salmonella biofilm formation, chronic infection, and immunity within the intestine and hepatobiliary tract. Frontiers in cellular and infection microbiology, 10, 624622.
Stepanović, S., Cirković, I., Ranin, L., & Svabić-Vlahović, M. (2004). Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Letters in applied microbiology, 38(5), 428–432. https://doi.org/10.1111/j.1472-765X.2004.01513.x
Ngogo, F. A., Joachim, A., Abade, A. M., Rumisha, S. F., Mizinduko, M. M., & Majigo, M. V. (2020). Factors associated with Salmonella infection in patients with gastrointestinal complaints seeking health care at Regional Hospital in Southern Highland of Tanzania. BMC infectious diseases, 20(1), 135. https://doi.org/10.1186/s12879-020-4849-7
Pinedo, L. C., Mughini-Gras, L., Franz, E., Hald, T., & Pires, S. M. (2022). Sources and trends of human salmonellosis in Europe, 2015–2019: An analysis of outbreak data. International Journal of Food Microbiology, 379, 109850.
Vidaković, S., Vranešević, J., Knežević, S., Pelić, M., Ružić, S., Pajić, M., & Pelić Ljubojević D. (2020). Microbiological property of raw meat imports in the year 2017. Veterinary Journal of Republic Srpska (Banja Luka), 20(1-2):118–124. doi: 10.7251/VETJEN2001118V
Jaki Tkalec, V., Furmeg, S., Bukvić, M., Cvetnić, M., Sokolović, J., Mustapić, P., Sokolić, K., Mikulić, M., & Cvetnić, Ž. (2021). Frequency of Salmonella spp. serovars in poultry meat in northwest Croatia. Journal Veterinarska Stanica, 52(4):387-396. doi:10.46419/vs.52.4.11
European Food Safety Authority, & European Centre for Disease Prevention and Control (2021). The European Union One Health 2020 Zoonoses Report. EFSA journal. European Food Safety Authority, 19(12), e06971. https://doi.org/10.2903/j.efsa.2021.6971.
Aljicevic. M., Cikotic. A., Bektas. S., Vranic. SM., Rebic. V., Abduzaimovic. A., Cemerlic. A. (2019). Antibiotic susceptibility/resistance among Salmonella species isolated in non-hospitalised patients in the canton of Sarajevo. J IMAB, 25(2): 2532-2536. doi: 10.5272/jimab.2019252.2532
Zdravstveno stanje stanovništva Republike Srpske 2021, JZU Institut za javno zdravstvo Republike Srpske, Banja Luka 2021.
Šefcová, M. A., Ortega-Paredes, D., Larrea-Álvarez, C. M., Mina, I., Guapás, V., Ayala-Velasteguí, D., Leoro-Garzón, P., Molina-Cuasapaz, G., Vinueza-Burgos, C., Revajová, V., & Larrea-Álvarez, M. (2023). Effects of Lactobacillus fermentum Administration on Intestinal Morphometry and Antibody Serum Levels in Salmonella-Infantis-Challenged Chickens. Microorganisms, 11(2), 256. https://doi.org/10.3390/microorganisms11020256
Drauch, V., Mitra, T., Liebhart, D., Hess, M., & Hess, C. (2022). Infection dynamics of Salmonella Infantis vary considerably between chicken lines. Avian Pathology, 51(6), 561–573. https://doi.org/10.1080/03079457.2022.2108373
Speranza, B., Corbo, M. R., & Sinigaglia, M. (2011). Effects of nutritional and environmental conditions on Salmonella sp. biofilm formation. Journal of food science, 76(1), M12–M16. https://doi.org/10.1111/j.1750-3841.2010.01936.x
Piras, F., Fois, F., Consolati, S. G., Mazza, R., & Mazzette, R. (2015). Influence of Temperature, Source, and Serotype on Biofilm Formation of Salmonella enterica Isolates from Pig Slaughterhouses. Journal of food protection, 78(10), 1875–1878. https://doi.org/10.4315/0362-028X.JFP-15-085
Shatila, F., Yaşa, İ., & Yalçın, H. T. (2021). Biofilm Formation by Salmonella enterica Strains. Current microbiology, 78(4), 1150–1158. https://doi.org/10.1007/s00284-021-02373-4
Govaert, M., Smet, C., Baka, M., Janssens, T., & Impe, J. V. (2018). Influence of incubation conditions on the formation of model biofilms by Listeria monocytogenes and Salmonella Typhimurium on abiotic surfaces. Journal of applied microbiology, 10.1111/jam.14071. https://doi.org/10.1111/jam.14071
Borges, K. A., Furian, T. Q., Souza, S. N., Menezes, R., Tondo, E. C., Salle, C. T. P., Moraes, H.L.S., & Nascimento, V. P. (2018). Biofilm formation capacity of Salmonella serotypes at different temperature conditions. Pesquisa Veterinária Brasileira, 38(1), 71–76. doi:10.1590/1678-5150-pvb-4928.
Webber, B., Oliveira, A. P. D., Pottker, E. S., Daroit, L., Levandowski, R., Santos, L. R. D., Nascimento V.P., & Rodrigues, L. B. (2019). Salmonella Enteritidis forms biofilm under low temperatures on different food industry surfaces. Ciência Rural, 49, e20181022