1. Introduction
Biofilms are complex communities of microbes embedded in a self-produced matrix of extracellular polymeric substances (EPS) (Flemming et al., 2016). They are the predominant life forms of microorganisms and can be found in almost any environment (Flemming & Wuertz, 2019). They form on rocks, soils, plants and animals surfaces, in extreme environments such as hot springs, and on many man-made surfaces such as pipes and sinks.
While beneficial in some industrial settings (Philipp et al., 2024), biofilms pose a major challenge to human and animal health, food safety and water supply (Cámara et al., 2022; Sentenac et al., 2022).
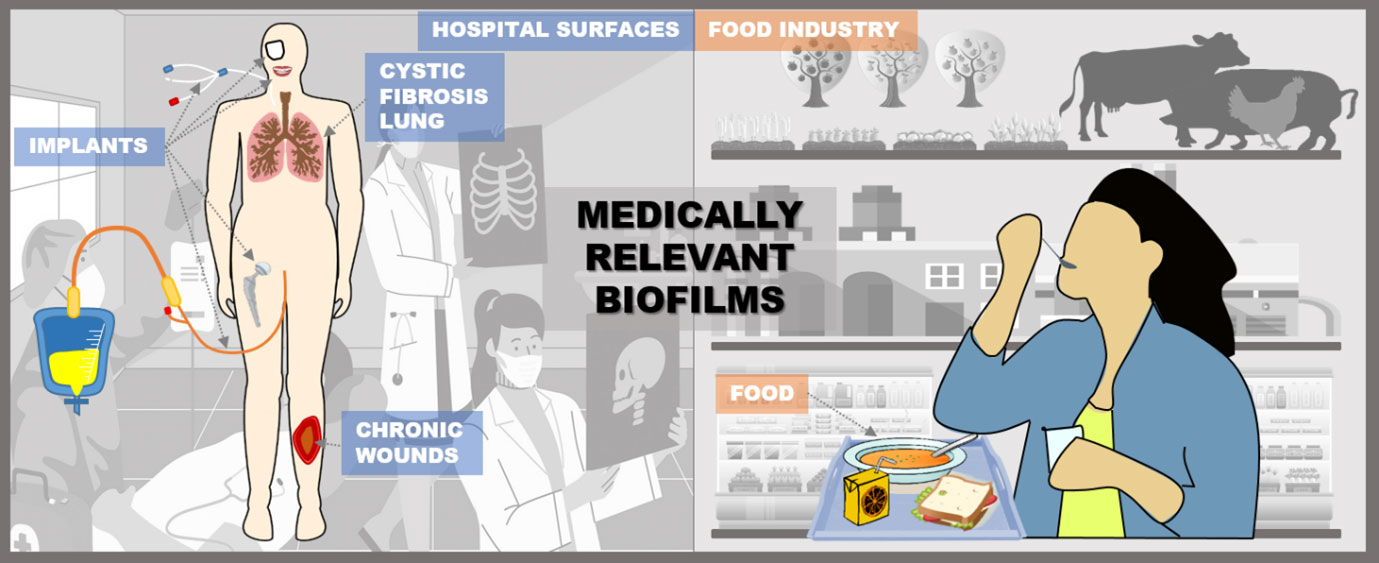
Medically relevant biofilms can develop on both biotic and abiotic surfaces (Fig. 1). They form on hospital surfaces, medical devices, patient tissues, and implants, as well as other non-medical surfaces (Percival, Suleman, et al., 2015). In addition, biofilms can exist as unattached aggregates and contaminate medical equipment, industrial facilities, and food processing plants. In food processing plants, biofilms can harbor pathogenic microorganisms that contaminate food products and pose a serious risk to food safety and public health.
Figure 1. Places of biofilm formation in medicine and the food industry where enzyme-based prevention strategies can be applied. Biofilms often form on abiotic surfaces in hospitals and food processing plants, where they can be inhibited by immobilizing enzymes on these surfaces or by incorporating them into disinfection treatments. In the human body, enzymes can prevent the formation of biofilms when immobilized on wound dressings and various implants or applied in soluble form. Food can be protected by enzyme-functionalized packaging or preservative coatings.
The biggest health problem associated with biofilms is their high tolerance and resistance to antibiotics. Biofilm-associated infections are often persistent and difficult to treat, requiring prolonged and aggressive therapeutic strategies. Both in vitro and in vivo studies show that the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of clinical antibiotics are generally 10 to 1,000 times higher for biofilm bacteria than those for planktonic bacteria (Høiby et al., 2011). Achieving the effective MBC for biofilm eradication in vivo is challenging due to the toxicity and side effects of high-dose antibiotics. This increased biofilm tolerance and even resistance can be attributed to several factors (Ciofu et al., 2022). The EPS matrix, which consists of polysaccharides, proteins, lipids and extracellular DNA (eDNA) (Hobley et al., 2015) inhibits the diffusion of antimicrobial agents and thus reduces their effective concentration within the biofilm (Powell et al., 2021). In addition, the matrix can chemically inactivate some antimicrobial agents through complex formation, enzymatic degradation, or sacrificial reactions (Singh et al., 2017). However, the matrix alone is not the only factor; the slow growth rates of cells in biofilms also contribute to their tolerance. Cells in biofilms often enter a stationary phase or a viable-but-nonculturable state (VBNC), which makes them less susceptible to antibiotics that target actively dividing cells (Ayrapetyan et al., 2018; Stewart & Franklin, 2008). Moreover, the biofilm environment facilitates horizontal gene transfer, a process in which bacteria exchange genetic material (Metzger et al., 2022). This is particularly efficient in biofilms due to the high cell density and close proximity (Mah, 2012).
Due to the low sensitivity of biofilm bacteria to antibiotics, the treatment of infections involving biofilms is often difficult and frequently ineffective. While young biofilms are easier to eradicate, mature biofilms are particularly resistant to treatment, so early diagnosis is necessary for successful intervention (Hengzhuang et al., 2011). However, most clinical biofilm infections are diagnosed at a mature stage, making them difficult to eradicate with antibiotics and often requiring chronic suppressive therapy or management of recurrence (Høiby et al., 2011; Wu et al., 2015).
Research into the formation, structure and function of biofilms is progressing and aims to develop innovative strategies to combat biofilms. To develop effective approaches to biofilm elimination, current research efforts are focused on the discovery of new antimicrobial agents and surface coatings that can prevent biofilm formation and reduce the burden of biofilm-associated infections and contamination. To prevent biofilm formation, it is critical to prevent microbial colonization of surfaces, as colonization is the first step in biofilm development and the moment when the biofilm is most susceptible (P. Gupta et al., 2016). This review focuses on inhibition strategies for medically relevant biofilms based on enzymes that have been investigated over the last decade.
2. Biofilm formation
The formation of biofilms is a complex process consisting of three main phases: Attachment to the surface, maturation and dispersal (Sauer et al., 2022). In the attachment phase, the planktonic cells come into contact with a surface, which can be either biotic (e.g. skin or bone) or abiotic (e.g. medical devices such as urinary catheters or artificial joints). In tissue infections, the bacteria adhere to each other and form aggregates. This initial adhesion is weak and reversible and is influenced by surface properties and environmental conditions. With the help of pili and fimbriae, the adhesion of the bacteria to the surface then becomes much stronger. Once the bacteria begin to produce EPS, which enables the cells to adhere strongly to each other and/or to a surface, the adhesion becomes irreversible.
During the maturation phase, the attached bacteria begin to multiply and form microcolonies. EPS production increases, forming a scaffold that holds the growing community together and develops into three-dimensional structures. As the biofilm continues to grow and mature, it becomes more complex and structured, creating a microenvironment characterized by nutrient and oxygen gradients. This leads to physiological heterogeneity, with fast-growing cells on the surface and slow-growing, tolerant cells in the deeper layers. As a result, the biofilm becomes more resistant to antibiotics and environmental stress during this phase. Finally, in the dispersion phase, cells or cell clusters detach from the biofilm to colonize new surfaces. This process is triggered by environmental changes or a lack of nutrients.
Biofilm formation depends on various regulatory signals, including messengers such as cyclic di-guanylate (c-di-GMP), small non-coding regulatory RNA (sRNA) and small molecules called autoinducers (AIs) via quorum sensing (QS). The switch between planktonic and biofilm lifestyle is controlled by c-di-GMP (Römling et al., 2013). High c-di-GMP concentrations trigger the production of adhesins and matrix polysaccharides and inhibit various types of motility, thereby promoting biofilm formation. Conversely, low levels of c-di-GMP reduce the production of adhesins and exopolysaccharides and increase bacterial motility, leading to biofilm dispersal (for a comprehensive overview see Valentini & Filloux, 2016).
sRNAs regulate biofilm formation by modulating gene expression, often at the post-transcriptional level (Taylor et al., 2017; Thomason et al., 2012). sRNAs help bacteria adapt to environmental stresses such as nutrient starvation and antimicrobial agents by regulating stress response genes, thereby improving biofilm stability and resistance. In addition, sRNAs influence the synthesis of extracellular polymeric substances (EPS), which are important components of the biofilm matrix, and regulate genes involved in biofilm dispersal, allowing the bacteria to return to a planktonic state when conditions change. Through these mechanisms, sRNAs control the complex processes underlying biofilm formation, maintenance and dispersal. The role of sRNA in biofilm formation has recently been reviewed (Mitra & Mukhopadhyay, 2023)
QS is a cell-to-cell communication system that enables bacteria to make collective decisions based on cell density and behave like a multicellular organism. A QS system consists of AIs, synthases that produce the AI, and a receptor/transcriptional regulator that recognizes the signal. When the AI binds to its receptor, it activates gene transcription, including AI biosynthesis, creating a positive feedback loop. At low cell density, AI levels are low and the receptor is only marginally active. With increasing cell density, the AI concentration reaches a threshold value that leads to complete activation of the receptor and upregulation of the target genes. Gram-positive and Gram-negative bacteria use different types of AIs. Gram-positive bacteria produce short oligopeptides as AIs, while signaling molecules of Gram-negative bacteria are small molecules such as acyl homoserine lactones (AHLs), alkylquinolones, thiazole compounds, α-hydroxyketones and diffusible signaling factors (fatty acid-like compounds) (Senerovic et al., 2020).
Some bacteria can use a single type of AI, while other species use QS system consisting of multiple pathways regulated by structurally different AIs. Pseudomonas aeruginosa, for example, uses four QS signaling pathways known as Las, Rhl, Pseudomonas Qunolone Signaling (PQS) and IQS (Papenfort & Bassler, 2016). The QS signaling pathways alone or interconnected regulate genes commonly associated with pathogenicity and influence virulence factor production, biofilm formation and various types of motility (Rutherford & Bassler, 2012).
The QS play a species-specific role in biofilm formation and influence the structural development and stabilization of the biofilm. In Gram-negative bacteria (e.g. P. aeruginosa and Burkholderia cenocepacia), QS is involved in the release of significant amounts of eDNA at later stages of biofilm development by autolysis of a bacterial subpopulation (Allesen-Holm et al., 2006; Pakkulnan et al., 2019). In P. aeruginosa, QS also regulates the synthesis of rhamnolipids, which are crucial for the late stages of biofilm development. Rhamnolipids maintain channels in mushroom-shaped biofilm structures, ensuring the proper distribution of nutrients and oxygen and the removal of waste products (Davey et al., 2003). Overproduction of rhamnolipids causes the biofilm to detach and spread (Boles et al., 2005). In addition, QS controls the production of LecA and LecB lectins (Winzer et al., 2000) and siderophores such as pyoverdin and pyochelin (Popat et al., 2017). These siderophores are involved in iron metabolism, with both low and high concentrations of iron inhibiting biofilm formation (Banin et al., 2005). In biofilms, AIs can reach much higher concentrations compared to environment of free-living organisms, which increases their effectiveness. The biofilm matrix itself can bind to and concentrate signaling molecules, enabling more effective QS (Keller & Surette, 2006).
3. Strategies to prevent medically relevant biofilms
Biofilm-forming microorganisms are responsible for 60-80% of human infections, particularly in healthcare settings, with the most problematic being methicillin-resistant Staphylococcus aureus (MRSA) and P. aeruginosa. These bacteria can form biofilms on medical devices like catheters and prosthetic joints, as well as in the lungs of cystic fibrosis patients and chronic wounds, leading to persistent, hard-to-treat infections. Other notable biofilm formers include uropathogenic Escherichia coli, which can cause chronic urinary tract infections, Enterococcus faecalis, a common cause of hospital-acquired infections such as endocarditis, Klebsiella pneumoniae, which poses serious risks to immunocompromised patients, and Streptococcus mutans, a major contributor to dental plaque and caries. The presence of biofilms enhances bacterial resistance to the immune system and antibiotics, complicating treatment strategies for these infections.
The most common foodborne pathogens that form biofilms include Salmonella spp. which cause salmonellosis and can adhere to various surfaces in food processing facilities, leading to significant health risks. E. coli, especially enterohemorrhagic strains such as O157, can form biofilms on food and food contact surfaces, leading to serious foodborne illness. Listeria monocytogenes is notorious for forming biofilms in cold and damp food processing facilities, leading to listeriosis, a serious infection with a high mortality rate. Campylobacter jejuni, a major cause of bacterial gastroenteritis, can form biofilms on poultry and other surfaces, contributing to food contamination. S. aureus and Bacillus cereus can form biofilms on food and surfaces and produce toxins that cause food poisoning. These pathogens pose a major challenge in food processing as they easily adhere to surfaces and form biofilms that resist cleaning and hygiene measures, leading to persistent contamination and food safety issues (Galié et al., 2018).
The strategies to inhibit biofilm formation are based on the two types of biofilm inhibitors with different modes of action:
1) Bactericidal agents: these molecules prevent biofilm formation by inhibiting bacterial growth. Common bactericidal biofilm inhibitors include novel small molecules and structural analogs of clinical antibiotics (Blasco et al., 2024; Kokot et al., 2023), metal complexes (Glišić et al., 2016; Rinehart et al., 2023; Savić et al., 2016; Sovari et al., 2021), nanoparticles (A. Gupta et al., 2018), plant derived molecules, extracts and essential oils (Gómez-Sequeda et al., 2020; Sánchez et al., 2016; Zizovic et al., 2018), antimicrobial peptides (Klubthawee et al., 2023), bacteriophages (Vukotic et al., 2020), and probiotics that produce antimicrobial metabolites (Al-Shamiri et al., 2023; Lee et al., 2021). While they are effective in the early stages of infection, prolonged use of bactericidal agents can lead to resistance that compromises the effectiveness of treatment.
2) Antivirulence molecules, in contrast to bactericidal agents, specifically target the virulence properties of pathogens, including biofilm formation, without interfering with bacterial growth. This makes antivirulence strategies particularly beneficial for the treatment of infections that are prone to resistance, as they pose a lower risk of resistance development.
Effective prevention of biofilm formation with antivirulence agents can be achieved by targeting different stages of the biofilm formation process.
- a) Bacterial adhesion to surfaces can be interrupted by the use of biosurfactants (Aleksic et al., 2017; Amirinejad et al., 2022; Sambanthamoorthy et al., 2014) such as glycolipids, rhamnolipids, lipopeptides, polysaccharide-protein complexes, phospholipids or fatty acids, antibodies targeting adhesion proteins such as pili or fimbriae (de Freitas et al., 2021; D. Sun et al., 2005), or chelators that can remove cations (Fe, Ca2+ and Mg2+) that play a role in microbial adhesion and biofilm formation (Abraham et al., 2012; O’May et al., 2009).
- b) Inhibition of QS and c-di-GMP signaling pathways
Quorum sensing can be disrupted at multiple points within the signaling pathway. Approaches include inhibiting the synthesis of autoinducers (AIs) using small molecules that target synthases (Chang et al., 2014), using quorum quenching (QQ) enzymes to hydrolyze AIs to reduce their concentration (Djokic et al., 2022; Koch et al., 2014; Malešević et al., 2020; Pustelny et al., 2009), and blocking the interaction between AIs and their receptors by competitive inhibition with naturally derived, synthetic or semi-synthetic molecules that are structurally similar to natural AIs (Aleksic et al., 2018, 2019; Aleksić et al., 2017; Pekmezovic et al., 2016).
c-di-GMP signaling can be modulated by small molecule inhibitors of c-di-GMP synthesis (Andersen et al., 2021; Chua et al., 2015) or by direct reduction of c-di-GMP levels with c-di-GMP sequestering peptides (Hee et al., 2020).
3) Inhibition of biofilm maturation can be achieved by blocking the biosynthetic pathways of extracellular polymeric substances (EPS) with small molecules (Razvi et al., 2023) or by hydrolyzing EPS components using specific enzymes (Atanaskovic et al., 2024; Baker et al., 2016; Banar et al., 2016; Lim et al., 2019).
Biofilm inhibitors can be applied directly by applying the agents to the site of infection or indirectly by functionalizing materials such as surfaces, medical devices and wound dressings to prevent biofilm formation.
4. Enzyme-based strategies to inhibit biofilm formation
Enzyme -based strategies offer a targeted method to combat biofilms as they are highly specific for biofilm components or signaling molecules, which helps to reduce off-target effects. The use of anti-biofilm enzymes carries no risk of resistance development as they act on extracellular compounds and not directly on bacterial cells. As they do not have a bactericidal effect, they should be used in combination with antibacterial agents to achieve maximum efficacy. In combination with antibiotics, enzymes can increase the efficacy of drugs by breaking down biofilm barriers or inhibiting bacterial virulence factors (Borges et al., 2020). However, enzyme-based therapies face challenges such as the instability of enzymes under certain conditions, high production costs and the need to combine them with other treatments (antibiotics or disinfectants). There is also a risk of immune reactions against the enzymes (Ghosh et al., 2019).
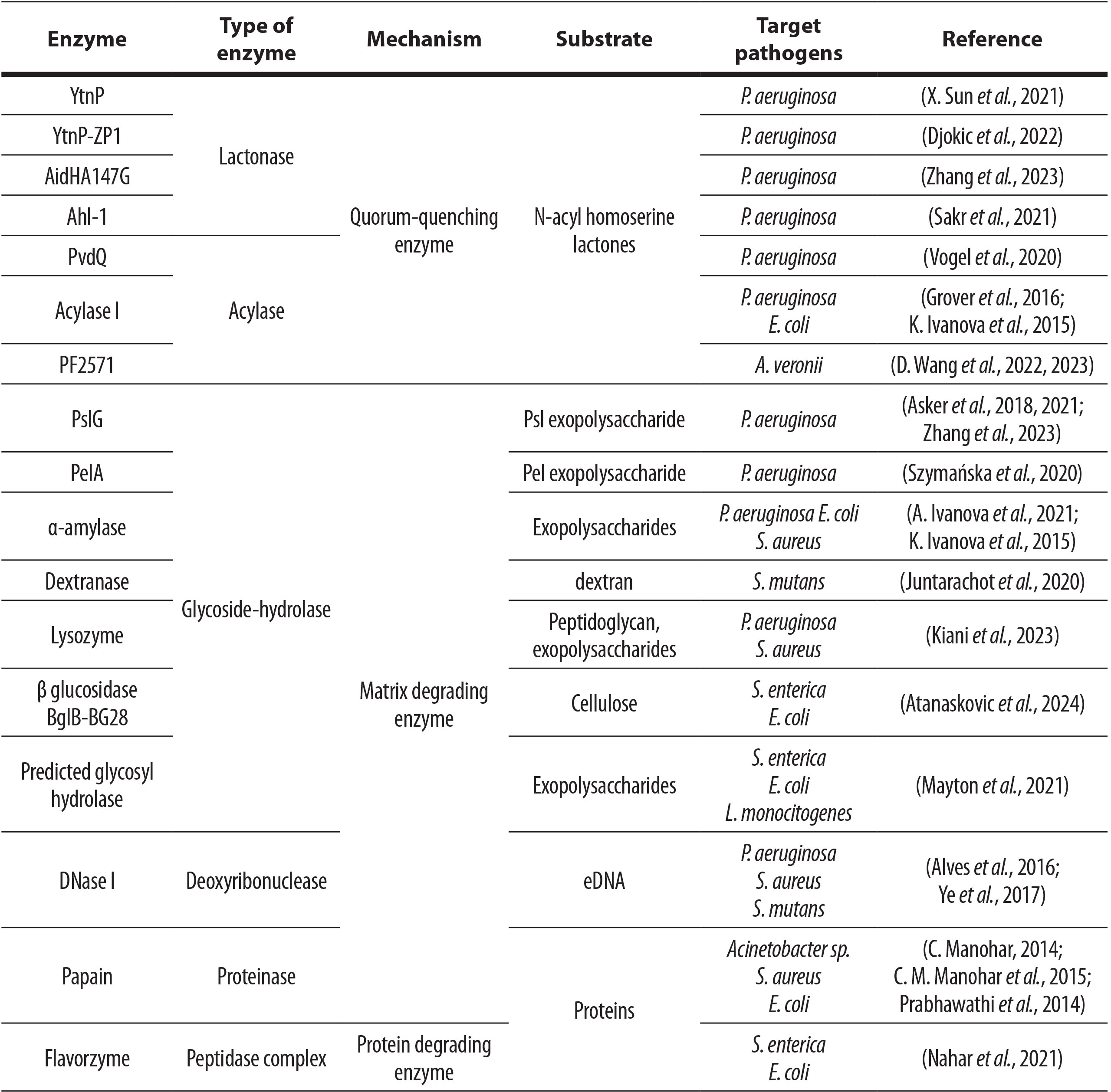
Enzyme-based inhibition of biofilm formation is achieved either by disrupting signaling cascades within and between microorganisms or by interfering with the synthesis of biofilm matrix components (Nahar et al., 2018). Here we discussed different types of enzymes including QQ enzymes, cyclic di-GMP degrading enzymes, exopolysaccharide degrading enzymes, proteolytic enzymes, oxidative enzymes, deoxyribonucleases, and lipolytic enzymes with potential to prevent formation of various medically relevant biofilms. However, most research in this field has focused on QQ enzymes and glucoside hydrolases, with P. aeruginosa being the most frequently studied pathogen (Table 1). This review presents the current state of the field and identifies research gaps that could be filled by studying other enzyme types and a broader range of pathogens.
Table 1. Summary of enzymes with proposed application in biofilm prevention, described in last 10 years
4.1 Application of anti-biofilm enzymes in healthcare
Biofilm formation on hospital surfaces poses a significant threat to patient safety and health outcomes. Biofilms can form on the surfaces of medical devices, frequently touched areas, and medical instruments (Assefa & Amare, 2022). These biofilms are notoriously difficult to eliminate and are associated with a high prevalence of hospital-acquired infections, accounting for over 65% of these cases (Preda & Săndulescu, 2019). The presence of biofilms highlights the urgent need for effective cleaning and disinfection protocols to reduce the risk of infection transmission in healthcare facilities.
Contamination of water lines poses a significant risk of microbial transfer to hospital surfaces, contributing to the potential spread of infections among patients and healthcare providers (Suleyman et al., 2018). Sun and colleagues developed a water filtration system to simulate biofilm contamination on water filters in dental unit water lines, highlighting the health risks these biofilms pose to both patients and dentists (X. Sun et al., 2021). They tested the antifouling activity of YtnP lactonase by applying it to P. aeruginosa inoculated water, which was continuously flowed through the filter membrane. Successful degradation of N-acylhomoserine lactones inhibited EPS production and reduced biofilm formation, as well as virulence factors production such as pyocyanin and rhamnolipids, suggesting its potential as a novel disinfectant for dental units.
In a different approach, Asker and colleagues used surface adsorption and covalent attachment techniques to immobilize PslGh on glass, polydimethylsiloxane (PDMS), and polystyrene (PS) surfaces (Asker et al., 2018). It was achieved by covalently attaching a Psl-specific glycoside hydrolase (PslGh) to various chemically distinct surfaces using amine functionalization (APTMS) and glutaraldehyde binding, which effectively inhibited P. aeruginosa colonization. Over a period of 8 days, PslGh-modified surfaces showed a significant reduction in surface attachment and biofilm formation by P. aeruginosa, achieving a reduction of surface-associated bacteria by approximately 99.9% (approximately 3-log) compared to control surfaces that were either untreated or treated with an inactive enzyme.
Biofilms are not limited to colonizing abiotic surfaces, they can also thrive on biotic surfaces. These microbial communities can develop in the tissues of patients and lead to chronic infections that are difficult to treat. Some of the most common infections associated with biofilms are those that occur in chronic wounds, in the airways of cystic fibrosis patients, and in association with medical implants (Del Pozo, 2018).
4.1.1 Chronic wound-associated biofilms
When the skin, our natural protective barrier, is compromised, it leaves the body vulnerable to microorganisms from the patient’s own flora or the external environment. Initially, the host’s immune system can control these microbes, but if they adhere to the wound surface and begin to multiply, a biofilm can form. As the biofilm matures, it becomes increasingly resistant to both the immune response and antimicrobial treatments, complicating wound care and raising the risk of chronic infection (Percival, et al., 2015). Given that patients with chronic wounds often have underlying medical conditions that compromise their immune systems (Falanga et al., 2022), the majority of these wounds (~80%) become colonized with biofilms (Malone et al., 2017). Most of the chronic wounds biofilms are polymicrobial, but when single-species biofilms occur, they are most likely caused by Pseudomonas or Staphylococcus genera (Wolcott et al., 2016). Another condition that harbors a fruitful environment for biofilm development is burn injury. It makes a patient immunocompromised and susceptible to opportunistic pathogens such are P. aeruginosa, Acinetobacter baumanii and S. aureus (Maslova et al., 2021). The economic burden of wound management on the UK’s NHS in 2017/2018 was £8.3 billion (Guest et al., 2020). Venous ulcers affect up to 1% of the US population, and diabetic foot ulcers impact 15-25% of diabetics (Richmond et al., 2013). Biofilm-associated chronic wounds may lead to serious consequences such as sepsis or amputation (Eriksson et al., 2022). Diabetics face a tenfold higher incidence of lower limb amputation and a 5-year mortality rate of 40% after ulceration (Jupiter et al., 2015). Additionally, 75% of all deaths in burned patients are caused by wound infection (Maslova et al., 2021).
In view of all these statistics and clearly identified risk groups, biofilm prevention is an important step in wound care. Enzyme-functionalized dressings are an attractive strategy for biofilm control in open wounds (Roy et al., 2017). Over the past 10 years, researchers have used QQ enzymes lactonases and P. aeruginosa-specific glycoside hydrolases (PslG and PelA) to test this theory, focusing mainly on P. aeruginosa as a causative agent of wound infection. Zhang et al. conducted an in vivo study in rats with burns and surface infections using a combined enzyme therapy of N-acylhomoserine lactonase AidHA147G, glycoside hydrolase PslG and tobramycin (Zhang et al., 2023). This therapy targets quorum sensing molecules and the Psl exopolysaccharide of P. aeruginosa and was administered once daily between the surgical dressing and the wound surface. The combined enzymes enhanced the antibiotic effect, leading to a reduction in inflammation, tissue damage and bacterial counts. In addition, the combined enzymes showed certain therapeutic effects even without the use of antibiotics (Zhang et al., 2023). A similar effect was observed in infected zebrafish tail wounds (Djokic et al., 2022). P. aeruginosa infection was cleared within 2 days by using YtnP-ZP1 lactonase in combination with tobramycin. Another administration technique was used by dissolving the lactonase Ahl-1 in 5% carboxymethylcellulose hydrogel and applying it in a burnt mouse model (Sakr et al., 2021). The survival rate was 100 compared to a control group in which a survival rate of 20 was observed.
By binding enzymes to solid supports or encapsulating them in matrices, researchers and manufacturers are harnessing the catalytic power of enzymes while overcoming the challenges associated with their stability and reusability (Khan, 2021). In addition to immobilization in hydrogels, wound care can also benefit from enzymes immobilized on dressing material. Bacterial nanocellulose (BC) has been shown to be an ultrafine network for wound dressings as it provides a moist, biocompatible environment while acting as an effective physical barrier against external infection (Bilal & Iqbal, 2019). Szymańska et al. immobilized the hydrolytic domain of PelA (PelAh) on BC membranes by physical adsorption (Szymańska et al., 2020). This affected biofilm formation by disrupting the integrity of the biofilm matrix, making it easier for adherent cells to detach from the BC surface. They suggest that the developed method could be improved by incorporating specific glycoside hydrolases targeting other components of the biofilm matrix, which could create a synergistic effect leading to more effective biofilm eradication and reducing the necessary dosage of additional antimicrobial chemotherapeutics.
4.1.2 Cystic fibrosis-associated biofilms
Cystic fibrosis (CF) is a genetic disorder that primarily affects the lungs and digestive system and is characterized by the production of thick, sticky mucus that obstructs the airways and leads to severe respiratory complications. Cystic fibrosis is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which disrupt ion transport and lead to dehydrated mucus (Cutting, 2015). This mucus accumulation creates an environment that favors chronic bacterial infections, particularly with P. aeruginosa and S. aureus, and leads to persistent inflammation and lung damage (Cohen & Prince, 2012). Recombinant human DNase, commonly known as Dornase alfa, is a treatment option for cystic fibrosis patients that is mainly used to improve lung function and reduce the viscosity of mucus in the airways. This enzyme cleaves extracellular DNA that accumulates in the lungs due to inflammation and infection, especially from neutrophils (Shak, 1995), but also from bacterial biofilm matrix (Z. Wang et al., 2024). Although clinical studies up to 2006 showed that the consistent use of DNase can significantly reduce the occurrence of pulmonary exacerbations, slow down the deterioration of lung function and prevent bacterial infections (Frederiksen et al., 2006), no other enzyme with comparable properties has been used to date.
4.1.3 Medical implant-associated biofilms
Medical implants are artificial devices inserted into the body for diagnostic, therapeutic, or rehabilitative purposes. Most commonly used medical devices are neurological, dental, cardiovascular, gastrointestinal, urological, intravascular, orthopedic implants, contact lenses, breast implants, IUDs and penile implants (Caldara et al., 2022). Unfortunately, device-associated infections, often associated with biofilm formation, remain a significant global health problem with serious clinical and economic implications (Percival, Suleman, et al., 2015). Staphylococcus species are the most commonly isolated bacteria on most medical devices (Caldara et al., 2022). Gastrointestinal implants have the greatest diversity of colonizing microorganisms, including a high prevalence of Enterococci, Enterobacteriaceae, Bacillus spp. and Streptococci, in addition to Staphylococcus spp. (Dautle et al., 2003). Urological implants and intravascular devices are frequently colonized by E. faecalis, P. aeruginosa and K. pneumoniae (Holá et al., 2010; Schulze et al., 2021). In addition to these bacteria, urological implants are also frequently colonized with E. coli, Proteus mirabilis and Enterobacter sp. (Holá et al., 2010). Dental implants in particular harbor the most distinct microbial communities, including Fusobacterium spp, Porphyromonas gingivalis, Prevotella spp., Selenomonas spp., Staphylococcus spp. and Streptococcus spp .(Caldara et al., 2022).
As far as the functionalization of implant surfaces with anti-biofilm enzymes is concerned, most work in the last 10 years has been carried out on urinary and venous catheters. Researchers in this field have used amylase (broad-spectrum glycoside hydrolase), acylase (QQ enzyme), PslG (Psl-specific glycoside hydrolase) and DNase to functionalize catheter surfaces and prevent biofilm formation of various pathogens such as E. coli, S. aureus and P. aeruginosa.
Catheter-associated urinary tract infections (CAUTIs) account for over 40 % of hospital-acquired infections and more than 80 % of all urinary tract infections and represent a global health problem (Milo et al., 2019). These infections increase mortality and morbidity, prolong hospitalization, increase healthcare costs, require prolonged antibiotic therapy and increase the risk of antibiotic resistance (Tenke et al., 2017). To prolong the lifespan of urinary catheters, reduce the incidence of CAUTIs and curb antibiotic resistance, Ivanova and colleagues proposed an innovative anti-biofilm coating. They combined antibacterial zinc oxide nanoparticles (ZnO NPs) with the exopolysaccharide-degrading enzyme amylase and applied them to silicone urinary catheters in a one-step sonochemical process. This nano-enhanced coating successfully inhibited biofilm formation by E. coli and S. aureus by 80 % and 60 %, respectively, for up to seven days in an in vitro catheter bladder model with artificial urine recirculation. In vivo experiments in a rabbit model confirmed the results that coated catheters led to a lower incidence of bacteriuria and delayed the early onset of CAUTIs compared to untreated silicone catheters (Ivanova et al., 2021). Vogel and coworkers used PvdQ acylase to create a QQ surface on polydimethylsiloxane (PDMS) using electrostatic interactions. The immobilized acylase maintained its activity after coating and showed a 6-fold reduction of the AI level (3-oxo-C12) and a significant reduction of a P. aeruginosa biofilm on a coated PDMS in a biosensor setup compared to the same untreated material (Vogel et al., 2020). Acylase derived from Aspergillus melleus was immobilized on biomedical polyurethane coatings using a multi-point covalent immobilization technique (Grover et al., 2016). These acylase-containing coatings showed enzymatic activity and effectively catalyzed the hydrolysis of AIs such as N-butyryl-L-homoserine lactone (C4-LHL), N-hexanoyl-L-homoserine lactone (C6-LHL) and N-(3-oxododecanoyl)-L-homoserine lactone (3-oxo-C12-LHL). In biofilm inhibition tests, the immobilization of the acylase led to a reduction in biofilm formation by P. aeruginosa of about 60%. Scanning electron microscopy also showed that the acylase- containing coatings had significantly fewer bacterial cells than the control coatings without acylase. Importantly, the acylase-embedded coatings maintained 90% of their activity after being stored dry at 37 °C for 7 days and exhibited greater stability than the free enzyme under physiological conditions, including in artificial urine. A combination of amylase and acylase was utilized to coat silicone urinary catheters using a layer-by-layer deposition technique (Ivanova et al., 2015). In a static biofilm assay, catheters coated with the acylase in the top layer showed a 60% reduction of biofilms formed either by only P. aeruginosa or both P. aeruginosa and E. coli. The comparable results were observed in the physical model of a catheterized human bladder, while in vivo experiments on catheterized rabbits showed biofilm inhibition only at certain catheter segments depending on the position in the animal body.
Central venous catheters (CVCs) are also highly susceptible to microbial colonization and biofilm formation, posing a significant risk for hospital-acquired infections. The average incidence of CVC-associated bloodstream infections in the US is 5.3 per 1000 catheter days in the ICU, which equates to approximately 80,000 cases per year (Gominet et al., 2017). Asker and colleagues showed that PslGh can be uniformly immobilized on the inner surfaces of catheter tubing made of medical grade polyethylene (PE-100), polyurethane and polydimethylsiloxane (silicone) (Asker et al., 2021). PslGh was covalently bound to the activated surface. Under dynamic flow culture conditions, P. aeruginosa colonization and biofilm formation showed a 3-log reduction in bacterial counts in the first 11 days and a 2-log reduction by day 14 for PslGh-modified PE-100 catheters compared to untreated controls. This was transferred to a rat infection model. PslGh-modified PE-100 catheters showed an approximate 1.5-log reduction in colonization of the clinical P. aeruginosa strain after 24 hours. In addition, a bifunctional coating with antimicrobial and anti-biofilm properties was used against both S. aureus and P. aeruginosa (Alves et al., 2016). Using polydopamine coating technology, they co-immobilized the antimicrobial lipopeptide Palm and the enzyme DNase I, which is able to degrade extracellular DNA from the biofilm matrix of both pathogens.
Deoxyribonuclease I (DNase I) was immobilized on a titanium surface (Ti) using dopamine as a linking agent (Ye et al., 2017). Titanium is widely used in the medical field due to its exceptional properties such as biocompatibility, corrosion resistance and strength to weight ratio. Since the 1950s, it has been the material of choice for a variety of applications, such as cardiovascular devices, orthopedic and dental implants. Titanium’s ability to integrate with bone, known as osseointegration, allows it to bond effectively with human tissue and makes it ideal for joint replacement and fracture fixation (Balazic et al., 2007). The resulting DNase-I coating showed significant efficacy in preventing adhesion and biofilm formation of S. mutans and S. aureus over a 24-hour period (Ye et al., 2017). S. mutans is the most common pathogen in dental plaque, a form of biofilm that forms on surfaces in the oral cavity. Juntarachot et al. developed a toothpaste with dextranase encapsulated in alginate beads with an effective dose of 4.5 units/g (Juntarachot et al., 2020). Brushing movements mechanically released the dextranase from the beads, which remained active for a prolonged period after brushing and are intended to inhibit biofilm formation by dissolving dextrans from the biofilm matrix.
Bandage contact lenses tend to accumulate bacterial biofilms during wear, with the two most common pathogens being P. aeruginosa and S. aureus (Zhu et al., 2019). Since lysozyme occurs naturally in tears, Kiani et al. investigated the effect of lysozyme on biofilm formation on bandage contact lenses by these two pathogens (Kiani et al., 2023). Lysozyme is a glycoside hydrolase that primarily targets glycosidic bonds in the peptidoglycan, but is also capable of destroying the biofilm matrix. It was physically adsorbed to the silicone hydrogel bandage contact lenses and inhibited the biofilm formation of 38.3% and 62.7% of P. aeruginosa and S. aureus, respectively. These results suggested that bandage contact lenses functionalized with lysozyme may reduce the risk of ocular infection after ocular surgery.
4.2. Application of anti-biofilm enzymes in food industry
Pathogenic biofilms in the food industry pose a significant health risk and an economic challenge. The biofilms formed by various foodborne pathogens such as B. cereus, S. aureus, L. monocytogenes, E. coli and Salmonella enterica, can develop on food contact surfaces and equipment and form a persistent reservoir of contamination (Galié et al., 2018). The presence of biofilms not only increases the likelihood of foodborne disease outbreaks, which account for around 60% of such incidents, but also complicates cleaning and hygiene measures due to their increased resistance to antimicrobial agents and physical removal methods (Liu et al., 2023). In the last 10 years, scientists have used anti-biofilm enzymes (β-glucosidase, flavorzymes, predicted glycoside hydrolase) in surface disinfection protocols targeting the structural components of biofilm. Atanaskovic and coworkers demonstrated the inhibitory effect of the β-glucosidase BglB-BG28 on Salmonella and E. coli biofilms on plastic, glass and metal surfaces (Atanaskovic et al., 2024). The enzyme targeted the synthesis process of cellulose fibers, one of the main components of Salmonella and E. coli biofilms. It was compatible with non-ionic detergents and showed a synergistic effect when subsequently treated with Oxicid S, a disinfectant commonly used in food processing plants and animal enclosures. The biofilm formation of Salmonella and E. coli was also successfully inhibited using a commercial peptidase, Flavorzyme (Nahar et al., 2021). The suggested mechanism is the inhibition of bacterial self-defense mechanisms by interfering with cellular proteins. Mayton et al had success in inhibiting biofilm formation, not only of Gram-negative Salmonella and E. coli, but also Gram-positive L. monocytogenes (Mayton et al., 2021). They used a parallel-plate flow chamber to directly observe the adhesion and detachment of the cells from the surface with a fluorescence microscope, thus simulating the real rinsing process. The enzyme was a predicted glycosyl hydrolase, and they proposed the degradation of exopolysaccharides as the mechanism.
4.2.1 Food-associated biofilms
Recent studies have explored innovative approaches to improve food safety through antimicrobial packaging to address the important issue of food contamination during processing, which contributes to spoilage and foodborne illness. The most commonly used enzyme for food packaging functionalization in the last 10 years has been papain, a proteolytic enzyme capable of destroying the structural components of biofilm. One such approach is the use of papain immobilized on polyurethane with the help of glutaraldehyde. This food packaging significantly reduced S. aureus biofilm and bacterial contamination of cottage cheese stored at 4°C for 7 days and showed superior antimicrobial activity compared to unmodified polymer films (Manohar, 2014). Papain was also immobilized on other materials, including low-density polyethylene (LDPE), high density polyethylene (HDPE), linear low density polyethylene (LLDPE) and polycaprolactam (PCL) using curcumin as a photocrosslinker (Manohar et al., 2015). The immobilized enzyme retained more than 90% of its activity after 30 days, with LLDPE showing the best biofilm properties against Acinetobacter sp. and S. aureus. This method effectively reduced microbial contamination of meat wrapped in modified LDPE and stored at 4°C for 7 days. In addition, papain-functionalized polycaprolactam was tested as a packaging material against E. coli biofilm. This material effectively inhibited biofilm growth and showed a drastic reduction in bacterial counts over 30 days (Prabhawathi et al., 2014). Another approach is to combat food spoilage through targeted bacterial QS. Wang et al. investigated the changes in the microbiota of red bream fillets during cold storage and found that the microbial diversity shifted in favor of Aeromonas veronii on the fourth day of storage (Wang et al., 2022). They proposed a strategy to alter the microbiota using the QQ acylase PF2571. The enzyme effectively inhibited spoilage-related QS factors, including biofilm formation, motility, protease, lipase and alginate production. The treated fillets maintained their quality for a longer period of time than the untreated controls, highlighting the potential for extending the shelf life of fish and fishery products. Next, Wang and colleagues developed a tamarind polysaccharide-polyvinyl alcohol hydrogel for seafood preservation (Wang et al., 2023). This hydrogel exhibited self-healing properties and demonstrated pH-dependent release of the QQ acylase PF2571. In vivo tests showed that hydrogel-coated fish fillets had a shelf life extended by more than three days.
5. Conclusion
The application of anti-biofilm enzymes in the healthcare and food industries represents a promising opportunity to combat the ongoing challenge of biofilm-associated infections and contamination. Biofilms, known for their resilience and contribution to hospital-acquired infections, significantly impact patient safety and healthcare outcomes. The integration of enzymes such as lactonases, acylases, glycoside hydrolases, DNases and proteases into cleaning protocols, medical devices, wound dressings and even food packaging offers a novel and targeted approach to biofilm prevention and elimination. In healthcare, the use of these enzymes to disrupt biofilm formation on medical devices and chronic wounds has shown significant potential. Enzyme-functionalized dressings and coatings on medical implants have demonstrated their ability to reduce bacterial colonization and biofilm formation, thereby reducing the risks of chronic infections and device-related complications. In the food industry, anti-biofilm enzymes have been successfully used to improve surface sanitization and increase food safety through antimicrobial packaging. These enzymes target the structural components of biofilm, reducing the risk of foodborne disease outbreaks and extending the shelf life of perishable goods. The ongoing development and refinement of enzyme-based approaches holds great promise for improving health practices and food safety. However, further research is needed to optimize these applications and ensure their efficacy and stability in different environments. By harnessing the catalytic power of enzymes, we can develop more effective, sustainable solutions to biofilm-related challenges in different sectors.
Funding: The authors have been funded by the Ministry of Science, Technological Development and Innovation, The Republic of Serbia (grant number 451-03-66/2024-03/200042).
References:
Abraham, N. M., Lamlertthon, S., Fowler, V. G., & Jefferson, K. K. (2012). Chelating agents exert distinct effects on biofilm formation in Staphylococcus aureus depending on strain background: Role for clumping factor B. Journal of Medical Microbiology, 61(8), 1062–1070. https://doi.org/10.1099/jmm.0.040758-0
Aleksic, I., Jeremic, J., Milivojevic, D., Ilic-Tomic, T., Šegan, S., Zlatović, M., Opsenica, D. M., & Senerovic, L. (2019). N-benzyl derivatives of long-chained 4-amino-7-chloro-quionolines as inhibitors of pyocyanin production in Pseudomonas aeruginosa. ACS Chemical Biology, 14(12), 2800–2809. https://doi.org/10.1021/acschembio.9b00682
Aleksic, I., Petkovic, M., Jovanovic, M., Milivojevic, D., Vasiljevic, B., Nikodinovic-Runic, J., & Senerovic, L. (2017). Anti-biofilm properties of bacterial di-rhamnolipids and their semi-synthetic amide derivatives. Frontiers in Microbiology, 8. https://doi.org/10.3389/fmicb.2017.02454
Aleksic, I., Ristivojevic, P., Pavic, A., Radojević, I., Čomić, L. R., Vasiljevic, B., Opsenica, D., Milojković-Opsenica, D., & Senerovic, L. (2018). Anti-quorum sensing activity, toxicity in zebrafish (Danio rerio) embryos and phytochemical characterization of Trapa natans leaf extracts. Journal of Ethnopharmacology, 222, 148–158. https://doi.org/10.1016/j.jep.2018.05.005
Aleksić, I., Šegan, S., Andric, F., Zlatovi, M., Moric, I., Opsenica, D. M., & Senerovic, L. (2017). Long-chained 4-aminoquinolines as Quorum Sensing inhibitors in Serratia marcescens and Pseudomonas aeruginosa. ACS Chemical Biology, 12(5), 1425–1434. https://doi.org/10.1021/acschembio.6b01149
Allesen-Holm, M., Barken, K. B., Yang, L., Klausen, M., Webb, J. S., Kjelleberg, S., Molin, S., Givskov, M., & Tolker-Nielsen, T. (2006). A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Molecular Microbiology, 59(4), 1114–1128. https://doi.org/10.1111/j.1365-2958.2005.05008.x
Al-Shamiri, M. M., Wang, J., Zhang, S., Li, P., Odhiambo, W. O., Chen, Y., Han, B., Yang, E., Xun, M., Han, L., & Han, S. (2023). Probiotic Lactobacillus species and their biosurfactants eliminate Acinetobacter baumannii biofilm in various manners. Microbiology Spectrum, 11(2). https://doi.org/10.1128/spectrum.04614-22
Alves, D., Magalhães, A., Grzywacz, D., Neubauer, D., Kamysz, W., & Pereira, M. O. (2016). Co-immobilization of Palm and DNase I for the development of an effective anti-infective coating for catheter surfaces. Acta Biomaterialia, 44, 313–322. https://doi.org/10.1016/j.actbio.2016.08.010
Amirinejad, N., Shahriary, P., & Hassanshahian, M. (2022). Investigation of the synergistic effect of glycolipid biosurfactant produced by Shewanella algae with some antibiotics against planktonic and biofilm forms of MRSA and antibiotic resistant Acinetobacter baumannii. World Journal of Microbiology & Biotechnology, 39(2), 45. https://doi.org/10.1007/s11274-022-03492-1
Andersen, J. B., Hultqvist, L. D., Jansen, C. U., Jakobsen, T. H., Nilsson, M., Rybtke, M., Uhd, J., Fritz, B. G., Seifert, R., Berthelsen, J., Nielsen, T. E., Qvortrup, K., Givskov, M., & Tolker-Nielsen, T. (2021). Identification of small molecules that interfere with c-di-GMP signaling and induce dispersal of Pseudomonas aeruginosa biofilms. Npj Biofilms and Microbiomes, 7(1), 1–13. https://doi.org/10.1038/s41522-021-00225-4
Asker, D., Awad, T. S., Baker, P., Howell, P. L., & Hatton, B. D. (2018). Non-eluting, surface-bound enzymes disrupt surface attachment of bacteria by continuous biofilm polysaccharide degradation. Biomaterials, 167, 168–176. https://doi.org/10.1016/j.biomaterials.2018.03.016
Asker, D., Awad, T. S., Raju, D., Sanchez, H., Lacdao, I., Gilbert, S., Sivarajah, P., Andes, D. R., Sheppard, D. C., Howell, P. L., & Hatton, B. D. (2021). Preventing Pseudomonas aeruginosa biofilms on indwelling catheters by surface-bound enzymes. ACS Applied Bio Materials, 4(12), 8248–8258. https://doi.org/10.1021/acsabm.1c00794
Assefa, M., & Amare, A. (2022). Biofilm-associated multi-drug resistance in hospital-acquired infections: A review. Infection and Drug Resistance, 15, 5061–5068. https://doi.org/10.2147/IDR.S379502
Atanaskovic, M., Moric, I., Rokic, M. B., Djokic, A., Pantovic, J., Despotović, D., & Senerovic, L. (2024a). Inhibition of Salmonella Enteritidis adhesion and biofilm formation by β-glucosidase B from Microbacterium sp. BG28. Food Bioscience, 57, 103543. https://doi.org/10.1016/j.fbio.2023.103543
Ayrapetyan, M., Williams, T., & Oliver, J. D. (2018). Relationship between the viable but nonculturable state and antibiotic persister cells. Journal of Bacteriology, 200(20), e00249-18. https://doi.org/10.1128/JB.00249-18
Baker, P., Hill, P. J., Snarr, B. D., Alnabelseya, N., Pestrak, M. J., Lee, M. J., Jennings, L. K., Tam, J., Melnyk, R. A., Parsek, M. R., Sheppard, D. C., Wozniak, D. J., & Howell, P. L. (2016). Exopolysaccharide biosynthetic glycoside hydrolases can be utilized to disrupt and prevent Pseudomonas aeruginosa biofilms. Science Advances, 2(5), e1501632. https://doi.org/10.1126/sciadv.1501632
Balazic, M., Kopac, J., Jackson, M. J., & Ahmed, W. (2007). Review: Titanium and titanium alloy applications in medicine. International Journal of Nano and Biomaterials, 1(1), 3. https://doi.org/10.1504/IJNBM.2007.016517
Banar, M., Emaneini, M., Satarzadeh, M., Abdellahi, N., Beigverdi, R., Leeuwen, W. B. van, & Jabalameli, F. (2016). Evaluation of mannosidase and trypsin enzymes effects on biofilm production of Pseudomonas aeruginosa isolated from burn wound infections. PLOS ONE, 11(10), e0164622. https://doi.org/10.1371/journal.pone.0164622
Banin, E., Vasil, M. L., & Greenberg, E. P. (2005). Iron and Pseudomonas aeruginosa biofilm formation. Proceedings of the National Academy of Sciences, 102(31), 11076–11081. https://doi.org/10.1073/pnas.0504266102
Bilal, M., & Iqbal, H. M. N. (2019). Naturally-derived biopolymers: Potential platforms for enzyme immobilization. International Journal of Biological Macromolecules, 130, 462–482. https://doi.org/10.1016/j.ijbiomac.2019.02.152
Blasco, B., Jang, S., Terauchi, H., Kobayashi, N., Suzuki, S., Akao, Y., Ochida, A., Morishita, N., Takagi, T., Nagamiya, H., Suzuki, Y., Watanabe, T., Lee, H., Lee, S., Shum, D., Cho, A., Koh, D., Park, S., Lee, H., … Piddock, L. J. V. (2024). High-throughput screening of small-molecules libraries identified antibacterials against clinically relevant multidrug-resistant A. baumannii and K. pneumoniae. eBioMedicine, 102. 105073
Boles, B. R., Thoendel, M., & Singh, P. K. (2005). Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Molecular Microbiology, 57(5), 1210–1223. https://doi.org/10.1111/j.1365-2958.2005.04743.x
Borges, A., Meireles, A., Mergulhão, F., Melo, L., & Simões, M. (2020). Biofilm control with enzymes. In Recent Trends in Biofilm Science and Technology (pp. 249–271). Elsevier. https://doi.org/10.1016/B978-0-12-819497-3.00011-8
Caldara, M., Belgiovine, C., Secchi, E., & Rusconi, R. (2022). Environmental, microbiological, and immunological features of bacterial biofilms associated with implanted medical devices. Clinical Microbiology Reviews, 35(2), e00221-20. https://doi.org/10.1128/cmr.00221-20
Cámara, M., Green, W., MacPhee, C. E., Rakowska, P. D., Raval, R., Richardson, M. C., Slater-Jefferies, J., Steventon, K., & Webb, J. S. (2022). Economic significance of biofilms: A multidisciplinary and cross-sectoral challenge. NPJ Biofilms and Microbiomes, 8(1), 42. https://doi.org/10.1038/s41522-022-00306-y
Chang, C.-Y., Krishnan, T., Wang, H., Chen, Y., Yin, W.-F., Chong, Y.-M., Tan, L. Y., Chong, T. M., & Chan, K.-G. (2014). Non-antibiotic quorum sensing inhibitors acting against N-acyl homoserine lactone synthase as druggable target. Scientific Reports, 4(1), 7245. https://doi.org/10.1038/srep07245
Chua, S. L., Hultqvist, L. D., Yuan, M., Rybtke, M., Nielsen, T. E., Givskov, M., Tolker-Nielsen, T., & Yang, L. (2015). In vitro and in vivo generation and characterization of Pseudomonas aeruginosa biofilm–dispersed cells via c-di-GMP manipulation. Nature Protocols, 10(8), 1165–1180. https://doi.org/10.1038/nprot.2015.067
Ciofu, O., Moser, C., Jensen, P. Ø., & Høiby, N. (2022). Tolerance and resistance of microbial biofilms. Nature Reviews Microbiology, 20(10), 621–635. https://doi.org/10.1038/s41579-022-00682-4
Cohen, T. S., & Prince, A. (2012). Cystic fibrosis: A mucosal immunodeficiency syndrome. Nature Medicine, 18(4), 509–519. https://doi.org/10.1038/nm.2715
Cutting, G. R. (2015). Cystic fibrosis genetics: From molecular understanding to clinical application. Nature Reviews. Genetics, 16(1), 45–56. https://doi.org/10.1038/nrg3849
Dautle, M. P., Wilkinson, T. R., & Gauderer, M. W. L. (2003). Isolation and identification of biofilm microorganisms from silicone gastrostomy devices. Journal of Pediatric Surgery, 38(2), 216–220. https://doi.org/10.1053/jpsu.2003.50046
Davey, M. E., Caiazza, N. C., & O’Toole, G. A. (2003). Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. Journal of Bacteriology, 185(3), 1027–1036. https://doi.org/10.1128/JB.185.3.1027-1036.2003
de Freitas, S. B., Wozeak, D. R., Neto, A. S., Cardoso, T. L., & Hartwig, D. D. (2021). A hypothetical adhesin protein induces anti-biofilm antibodies against multi-drug resistant Acinetobacter baumannii. Microbial Pathogenesis, 159, 105112. https://doi.org/10.1016/j.micpath.2021.105112
Del Pozo, J. L. (2018). Biofilm-related disease. Expert Review of Anti-Infective Therapy, 16(1), 51–65. https://doi.org/10.1080/14787210.2018.1417036
Djokic, L., Stankovic, N., Galic, I., Moric, I., Radakovic, N., Šegan, S., Pavic, A., & Senerovic, L. (2022a). Novel Quorum Quenching YtnP lactonase from Bacillus paralicheniformis reduces Pseudomonas aeruginosa virulence and increases antibiotic efficacy in vivo. Frontiers in Microbiology, 13, 906312. https://doi.org/10.3389/fmicb.2022.906312
Eriksson, E., Liu, P. Y., Schultz, G. S., Martins‐Green, M. M., Tanaka, R., Weir, D., Gould, L. J., Armstrong, D. G., Gibbons, G. W., Wolcott, R., Olutoye, O. O., Kirsner, R. S., & Gurtner, G. C. (2022). Chronic wounds: Treatment consensus. Wound Repair and Regeneration, 30(2), 156–171. https://doi.org/10.1111/wrr.12994
Falanga, V., Isseroff, R. R., Soulika, A. M., Romanelli, M., Margolis, D., Kapp, S., Granick, M., & Harding, K. (2022). CHRONIC WOUNDS PRIMER. Nature Reviews. Disease Primers, 8(1), 50. https://doi.org/10.1038/s41572-022-00377-3
Flemming, H.-C., Wingender, J., Szewzyk, U., Steinberg, P., Rice, S. A., & Kjelleberg, S. (2016). Biofilms: An emergent form of bacterial life. Nature Reviews Microbiology, 14, 563–575. https://doi.org/10.1038/nrmicro.2016.94
Flemming, H.-C., & Wuertz, S. (2019). Bacteria and archaea on Earth and their abundance in biofilms. Nature Reviews. Microbiology, 17(4), 247–260. https://doi.org/10.1038/s41579-019-0158-9
Frederiksen, B., Pressler, T., Hansen, A., & Koch, C. (2006). Effect of aerosolized rhDNase (Pulmozyme†) on pulmonary colonization in patients with cystic fibrosis. Acta Pediatrica, 95, 1070–1074. https://doi.org/10.1080/08035250600752466
Galié, S., García-Gutiérrez, C., Miguélez, E. M., Villar, C. J., & Lombó, F. (2018). Biofilms in the food industry: Health aspects and control methods. Frontiers in Microbiology, 9. https://doi.org/10.3389/fmicb.2018.00898
Ghosh, S., Alam, S., Rathore, A. S., & Khare, S. K. (2019). Stability of therapeutic enzymes: challenges and recent advances. Advances in Experimental Medicine and Biology, 1148, 131–150. https://doi.org/10.1007/978-981-13-7709-9_7
Glišić, B. Đ., Senerovic, L., Comba, P., Wadepohl, H., Veselinovic, A., Milivojevic, D. R., Djuran, M. I., & Nikodinovic-Runic, J. (2016). Silver(I) complexes with phthalazine and quinazoline as effective agents against pathogenic Pseudomonas aeruginosa strains. Journal of Inorganic Biochemistry, 155, 115–128. https://doi.org/10.1016/j.jinorgbio.2015.11.026
Gómez-Sequeda, N., Cáceres, M., Stashenko, E. E., Hidalgo, W., & Ortiz, C. (2020). Antimicrobial and antibiofilm activities of essential oils against Escherichia coli O157:H7 and methicillin-resistant Staphylococcus aureus (MRSA). Antibiotics (Basel, Switzerland), 9(11), 730. https://doi.org/10.3390/antibiotics9110730
Gominet, M., Compain, F., Beloin, C., & Lebeaux, D. (2017). Central venous catheters and biofilms: Where do we stand in 2017? APMIS, 125(4), 365–375. https://doi.org/10.1111/apm.12665
Grover, N., Plaks, J. G., Summers, S. R., Chado, G. R., Schurr, M. J., & Kaar, J. L. (2016). Acylase‐containing polyurethane coatings with anti‐biofilm activity. Biotechnology and Bioengineering, 113(12), 2535–2543. https://doi.org/10.1002/bit.26019
Guest, J. F., Fuller, G. W., & Vowden, P. (2020). Cohort study evaluating the burden of wounds to the UK’s National Health Service in 2017/2018: Update from 2012/2013. BMJ Open, 10(12), e045253. https://doi.org/10.1136/bmjopen-2020-045253
Gupta, A., Landis, R. F., Li, C.-H., Schnurr, M., Das, R., Lee, Y.-W., Yazdani, M., Liu, Y., Kozlova, A., & Rotello, V. M. (2018). Engineered polymer nanoparticles with unprecedented antimicrobial efficacy and therapeutic indices against multidrug-resistant bacteria and biofilms. Journal of the American Chemical Society, 140(38), 12137–12143. https://doi.org/10.1021/jacs.8b06961
Gupta, P., Sarkar, S., Das, B., Bhattacharjee, S., & Tribedi, P. (2016). Biofilm, pathogenesis and prevention—a journey to break the wall: A review. Archives of Microbiology, 198(1), 1–15. https://doi.org/10.1007/s00203-015-1148-6
Hee, C.-S., Habazettl, J., Schmutz, C., Schirmer, T., Jenal, U., & Grzesiek, S. (2020). Intercepting second-messenger signaling by rationally designed peptides sequestering c-di-GMP. PNAS, 117(29), 17211–17220.
Hengzhuang, W., Wu, H., Ciofu, O., Song, Z., & Høiby, N. (2011). Pharmacokinetics/pharmacodynamics of colistin and imipenem on mucoid and nonmucoid Pseudomonas aeruginosa biofilms▿. Antimicrobial Agents and Chemotherapy, 55(9), 4469–4474. https://doi.org/10.1128/AAC.00126-11
Hobley, L., Harkins, C., MacPhee, C. E., & Stanley-Wall, N. R. (2015). Giving structure to the biofilm matrix: An overview of individual strategies and emerging common themes. FEMS Microbiology Reviews, 39(5), 649–669. https://doi.org/10.1093/femsre/fuv015
Høiby, N., Ciofu, O., Johansen, H. K., Song, Z., Moser, C., Jensen, P. Ø., Molin, S., Givskov, M., Tolker-Nielsen, T., & Bjarnsholt, T. (2011). The clinical impact of bacterial biofilms. International Journal of Oral Science, 3(2), 55–65. https://doi.org/10.4248/IJOS11026
Holá, V., Ruzicka, F., & Horka, M. (2010). Microbial diversity in biofilm infections of the urinary tract with the use of sonication techniques. FEMS Immunology & Medical Microbiology, 59(3), 525–528. https://doi.org/10.1111/j.1574-695X.2010.00703.x
Ivanova, A., Ivanova, K., Perelshtein, I., Gedanken, A., Todorova, K., Milcheva, R., Dimitrov, P., Popova, T., & Tzanov, T. (2021). Sonochemically engineered nano-enabled zinc oxide/amylase coatings prevent the occurrence of catheter-associated urinary tract infections. Materials Science and Engineering: C, 131, 112518. https://doi.org/10.1016/j.msec.2021.112518
Ivanova, K., Fernandes, M. M., Francesko, A., Mendoza, E., Guezguez, J., Burnet, M., & Tzanov, T. (2015). Quorum-Quenching and matrix-degrading enzymes in multilayer coatings synergistically prevent bacterial biofilm formation on urinary catheters. ACS Applied Materials & Interfaces, 7(49), 27066–27077. https://doi.org/10.1021/acsami.5b09489
Juntarachot, N., Sirilun, S., Kantachote, D., Sittiprapaporn, P., Tongpong, P., Peerajan, S., & Chaiyasut, C. (2020). Anti-Streptococcus mutans and anti-biofilm activities of dextranase and its encapsulation in alginate beads for application in toothpaste. PeerJ, 8, e10165. https://doi.org/10.7717/peerj.10165
Jupiter, D. C., Thorud, J. C., Buckley, C. J., & Shibuya, N. (2015). The impact of foot ulceration and amputation on mortality in diabetic patients. I: From ulceration to death, a systematic review. International Wound Journal, 13(5), 892–903. https://doi.org/10.1111/iwj.12404
Keller, L., & Surette, M. G. (2006). Communication in bacteria: An ecological and evolutionary perspective. Nature Reviews Microbiology, 4(4), 249–258. https://doi.org/10.1038/nrmicro1383
Khan, M. R. (2021). Immobilized enzymes: A comprehensive review. Bulletin of the National Research Centre, 45(1), 207. https://doi.org/10.1186/s42269-021-00649-0
Kiani, P., Soozanipour, A., Rezayat, A., & Taheri-Kafrani, A. (2023). Lysozyme-immobilized bandage contact lens inhibits the growth and biofilm formation of common eye pathogens in vitro. Experimental Eye Research, 234, 109601. https://doi.org/10.1016/j.exer.2023.109601
Klubthawee, N., Wongchai, M., & Aunpad, R. (2023). The bactericidal and antibiofilm effects of a lysine-substituted hybrid peptide, CM-10K14K, on biofilm-forming Staphylococcus epidermidis. Scientific Reports, 13(1), 22262. https://doi.org/10.1038/s41598-023-49302-y
Koch, G., Nadal-Jimenez, P., Reis, C. R., Muntendam, R., Bokhove, M., Melillo, E., Dijkstra, B. W., Cool, R. H., & Quax, W. J. (2014). Reducing virulence of the human pathogen Burkholderia by altering the substrate specificity of the quorum-quenching acylase PvdQ. PNAS, 111(4).
Kokot, M., Weiss, M., Zdovc, I., Senerovic, L., Radakovic, N., Anderluh, M., Minovski, N., & Hrast, M. (2023). Amide containing NBTI antibacterials with reduced hERG inhibition, retained antimicrobial activity against gram-positive bacteria and in vivo efficacy. European Journal of Medicinal Chemistry, 250, 115160. https://doi.org/10.1016/j.ejmech.2023.115160
Lee, J.-E., Lee, N.-K., & Paik, H.-D. (2021). Antimicrobial and anti-biofilm effects of probiotic Lactobacillus plantarum KU200656 isolated from kimchi. Food Science and Biotechnology, 30(1), 97–106. https://doi.org/10.1007/s10068-020-00837-0
Lim, E. S., Koo, O. K., Kim, M.-J., & Kim, J.-S. (2019). Bio-enzymes for inhibition and elimination of Escherichia coli O157:H7 biofilm and their synergistic effect with sodium hypochlorite. Scientific Reports, 9(1), Article 1. https://doi.org/10.1038/s41598-019-46363-w
Liu, X., Yao, H., Zhao, X., & Ge, C. (2023). Biofilm Formation and Control of Foodborne Pathogenic Bacteria. Molecules, 28(6), 2432. https://doi.org/10.3390/molecules28062432
Mah, T.-F. (2012). Biofilm-Specific Antibiotic Resistance. Future Microbiology, 7(9), 1061–1072. https://doi.org/10.2217/fmb.12.76
Malešević, M., Stanisavljević, N., Novović, K., Polović, N., Vasiljević, Z., Kojić, M., & Jovčić, B. (2020). Burkholderia cepacia YtnP and Y2-aiiA lactonases inhibit virulence of Pseudomonas aeruginosa via quorum quenching activity. Microbial Pathogenesis, 149, 104561. https://doi.org/10.1016/j.micpath.2020.104561
Malone, M., Bjarnsholt, T., McBain, A. J., James, G. A., Stoodley, P., Leaper, D., Tachi, M., Schultz, G., Swanson, T., & Wolcott, R. D. (2017). The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. Journal of Wound Care, 26(1), 20–25. https://doi.org/10.12968/jowc.2017.26.1.20
Manohar, C. (2014). Papain Immobilized Polyurethane Film as Antimicrobial Food Package.
Manohar, C. M., Prabhawathi, V., Sivakumar, P. M., & Doble, M. (2015). Design of a papain immobilized antimicrobial food package with curcumin as a crosslinker. PLOS ONE, 10(4), e0121665. https://doi.org/10.1371/journal.pone.0121665
Maslova, E., Eisaiankhongi, L., Sjöberg, F., & McCarthy, R. R. (2021). Burns and biofilms: Priority pathogens and in vivo models. NPJ Biofilms and Microbiomes, 7, 73. https://doi.org/10.1038/s41522-021-00243-2
Mayton, H. M., Walker, S. L., & Berger, B. W. (2021). Disrupting irreversible bacterial adhesion and biofilm formation with an engineered enzyme. Applied and Environmental Microbiology, 87(13). https://doi.org/10.1128/AEM.00265-21
Metzger, G. A., Ridenhour, B. J., France, M., Gliniewicz, K., Millstein, J., Settles, M. L., Forney, L. J., Stalder, T., & Top, E. M. (2022). Biofilms preserve the transmissibility of a multi-drug resistance plasmid. Npj Biofilms and Microbiomes, 8(1), 1–10. https://doi.org/10.1038/s41522-022-00357-1
Milo, S., Nzakizwanayo, J., Hathaway, H. J., Jones, B. V., & Jenkins, A. T. A. (2019). Emerging medical and engineering strategies for the prevention of long-term indwelling catheter blockage. Proceedings of the Institution of Mechanical Engineers. Part H, Journal of Engineering in Medicine, 233(1), 68–83. https://doi.org/10.1177/0954411918776691
Mitra, A., & Mukhopadhyay, S. (2023). Regulation of biofilm formation by non-coding RNA in prokaryotes. Current Research in Pharmacology and Drug Discovery, 4, 100151. https://doi.org/10.1016/j.crphar.2022.100151
Nahar, S., Jeong, H. L., Kim, Y., Ha, A. J., Roy, P. K., Park, S. H., Ashrafudoulla, Md., Mizan, Md. F. R., & Ha, S.-D. (2021). Inhibitory effects of Flavourzyme on biofilm formation, Quorum Sensing, and virulence genes of foodborne pathogens Salmonella Typhimurium and Escherichia coli. Food Research International, 147, 110461. https://doi.org/10.1016/j.foodres.2021.110461
Nahar, S., Mizan, Md. F. R., Ha, A. J., & Ha, S. (2018). Advances and future prospects of enzyme‐based biofilm prevention approaches in the food industry. Comprehensive Reviews in Food Science and Food Safety, 17(6), 1484–1502. https://doi.org/10.1111/1541-4337.12382
O’May, C. Y., Sanderson, K., Roddam, L. F., Kirov, S. M., & Reid, D. W. (2009). Iron-binding compounds impair Pseudomonas aeruginosa biofilm formation, especially under anaerobic conditions. Journal of Medical Microbiology, 58(Pt 6), 765–773. https://doi.org/10.1099/jmm.0.004416-0
Pakkulnan, R., Anutrakunchai, C., Kanthawong, S., Taweechaisupapong, S., Chareonsudjai, P., & Chareonsudjai, S. (2019). Extracellular DNA facilitates bacterial adhesion during Burkholderia pseudomallei biofilm formation. PLOS ONE, 14(3), e0213288. https://doi.org/10.1371/journal.pone.0213288
Papenfort, K., & Bassler, B. L. (2016). Quorum sensing signal–response systems in Gram-negative bacteria. Nature Reviews Microbiology, 14(9), 576–588. https://doi.org/10.1038/nrmicro.2016.89
Pekmezovic, M., Aleksic, I., Barac, A., Arsic-Arsenijevic, V., Vasiljevic, B., Nikodinovic-Runic, J., & Senerovic, L. (2016). Prevention of polymicrobial biofilms composed of Pseudomonas aeruginosa and pathogenic fungi by essential oils from selected Citrus species. Pathogens and Disease, 74. https://doi.org/10.1093/femspd/ftw102
Percival, S. L., McCarty, S. M., & Lipsky, B. (2015). Biofilms and wounds: An overview of the evidence. Advances in Wound Care, 4(7), 373–381. https://doi.org/10.1089/wound.2014.0557
Percival, S. L., Suleman, L., Vuotto, C., & Donelli, G. (2015). Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. Journal of Medical Microbiology, 64(4), 323–334. https://doi.org/10.1099/jmm.0.000032
Philipp, L.-A., Bühler, K., Ulber, R., & Gescher, J. (2024). Beneficial applications of biofilms. Nature Reviews Microbiology, 22(5), 276–290. https://doi.org/10.1038/s41579-023-00985-0
Popat, R., Harrison, F., da Silva, A. C., Easton, S. A. S., McNally, L., Williams, P., & Diggle, S. P. (2017). Environmental modification via a quorum sensing molecule influences the social landscape of siderophore production. Proceedings of the Royal Society B: Biological Sciences, 284(1852), 20170200. https://doi.org/10.1098/rspb.2017.0200
Powell, L. C., Abdulkarim, M., Stokniene, J., Yang, Q. E., Walsh, T. R., Hill, K. E., Gumbleton, M., & Thomas, D. W. (2021). Quantifying the effects of antibiotic treatment on the extracellular polymer network of antimicrobial resistant and sensitive biofilms using multiple particle tracking. Npj Biofilms and Microbiomes, 7(1), 1–11. https://doi.org/10.1038/s41522-020-00172-6
Prabhawathi, V., Boobalan, T., Sivakumar, P. M., & Doble, M. (2014). Functionalized polycaprolactam as an active food package for antibiofilm activity and extended shelf life. Colloids and Surfaces B: Biointerfaces, 123, 461–468. https://doi.org/10.1016/j.colsurfb.2014.09.041
Preda, V. G., & Săndulescu, O. (2019). Communication is the key: Biofilms, quorum sensing, formation and prevention. Discoveries, 7(3), e100. https://doi.org/10.15190/d.2019.13
Pustelny, C., Albers, A., Büldt-Karentzopoulos, K., Parschat, K., Chhabra, S. R., Cámara, M., Williams, P., & Fetzner, S. (2009). Dioxygenase-mediated quenching of quinolone-dependent quorum sensing in Pseudomonas aeruginosa. Chemistry & Biology, 16(12), 1259–1267. https://doi.org/10.1016/j.chembiol.2009.11.013
Razvi, E., DiFrancesco, B. R., Wasney, G. A., Morrison, Z. A., Tam, J., Auger, A., Baker, P., Alnabelseya, N., Rich, J. D., Sivarajah, P., Whitfield, G. B., Harrison, J. J., Melnyk, R. A., Nitz, M., & Howell, P. L. (2023). Small molecule inhibition of an exopolysaccharide modification enzyme is a viable strategy to block Pseudomonas aeruginosa Pel biofilm formation. Microbiology Spectrum, 11(3), e0029623. https://doi.org/10.1128/spectrum.00296-23
Richmond, N. A., Maderal, A. D., & Vivas, A. C. (2013). Evidence-based management of common chronic lower extremity ulcers: Management of chronic lower extremity ulcers. Dermatologic Therapy, 26(3), 187–196. https://doi.org/10.1111/dth.12051
Rinehart, N. I., Saunthwal, R. K., Wellauer, J., Zahrt, A. F., Schlemper, L., Shved, A. S., Bigler, R., Fantasia, S., & Denmark, S. E. (2023). Development and Validation of a Chemoinformatic Workflow for Predicting Reaction Yield for Pd-Catalyzed C-N Couplings with Substrate Generalizability. ChemRxiv. https://doi.org/10.26434/chemrxiv-2022-hspwv-v2
Römling, U., Galperin, M. Y., & Gomelsky, M. (2013). Cyclic di-GMP: The first 25 years of a universal bacterial second messenger. Microbiology and Molecular Biology Reviews : MMBR, 77(1), 1–52. https://doi.org/10.1128/MMBR.00043-12
Roy, R., Tiwari, M., Donelli, G., & Tiwari, V. (2017). Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence, 9(1), 522–554. https://doi.org/10.1080/21505594.2017.1313372
Rutherford, S. T., & Bassler, B. L. (2012). Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harbor Perspectives in Medicine, 2(11), a012427. https://doi.org/10.1101/cshperspect.a012427
Sakr, M. M., Elkhatib, W. F., Aboshanab, K. M., Mantawy, E. M., Yassien, M. A., & Hassouna, N. A. (2021). In vivo evaluation of a recombinant N-acylhomoserine lactonase formulated in a hydrogel using a murine model infected with MDR Pseudomonas aeruginosa clinical isolate, CCASUP2. AMB Express, 11, 109. https://doi.org/10.1186/s13568-021-01269-7
Sambanthamoorthy, K., Feng, X., Patel, R., Patel, S., & Paranavitana, C. (2014). Antimicrobial and antibiofilm potential of biosurfactants isolated from lactobacilli against multi-drug-resistant pathogens. BMC Microbiology, 14(1), 197. https://doi.org/10.1186/1471-2180-14-197
Sánchez, E., Rivas Morales, C., Castillo, S., Leos-Rivas, C., García-Becerra, L., & Ortiz Martínez, D. M. (2016). Antibacterial and antibiofilm activity of methanolic plant extracts against nosocomial microorganisms. Evidence-Based Complementary and Alternative Medicine, 2016(1), 1572697. https://doi.org/10.1155/2016/1572697
Sauer, K., Stoodley, P., Goeres, D. M., Hall-Stoodley, L., Burmølle, M., Stewart, P. S., & Bjarnsholt, T. (2022). The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nature Reviews Microbiology, 20(10), 608–620. https://doi.org/10.1038/s41579-022-00767-0
Savić, N. D., Glišić, B. Đ., Wadepohl, H., Pavic, A., Senerovic, L., Nikodinovic-Runic, J., & Djuran, M. I. (2016). Silver(I) complexes with quinazoline and phthalazine: Synthesis, structural characterization and evaluation of biological activities. MedChemComm, 7(2), 282–291. https://doi.org/10.1039/C5MD00494B
Schulze, A., Mitterer, F., Pombo, J. P., & Schild, S. (2021). Biofilms by bacterial human pathogens: Clinical relevance – development, composition and regulation – therapeutical strategies. Microbial Cell, 8(2), 28–56. https://doi.org/10.15698/mic2021.02.741
Senerovic, L., Moric, I., Milivojevic, D., & Opsenica, D. (2020). Nature-inspired synthetic analogues of quorum sensing signaling molecules as novel therapeutics against Pseudomonas aeruginosa infections. In M. Ozturk, D. Egamberdieva, & M. Pešić (Eds.), Biodiversity and Biomedicine (pp. 497–523). Academic Press. https://doi.org/10.1016/B978-0-12-819541-3.00025-6
Sentenac, H., Loyau, A., Leflaive, J., & Schmeller, D. S. (2022). The significance of biofilms to human, animal, plant and ecosystem health. Functional Ecology, 36(2), 294–313. https://doi.org/10.1111/1365-2435.13947
Singh, S., Singh, S. K., Chowdhury, I., & Singh, R. (2017). Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. The Open Microbiology Journal, 11, 53–62. https://doi.org/10.2174/1874285801711010053
Sovari, S. N., Radakovic, N., Roch, P., Crochet, A., Pavic, A., & Zobi, F. (2021). Combatting AMR: A molecular approach to the discovery of potent and non-toxic rhenium complexes active against C. albicans-MRSA co-infection. European Journal of Medicinal Chemistry, 226, 113858. https://doi.org/10.1016/j.ejmech.2021.113858
Stewart, P. S., & Franklin, M. J. (2008). Physiological heterogeneity in biofilms. Nature Reviews. Microbiology, 6(3), 199–210. https://doi.org/10.1038/nrmicro1838
Suleyman, G., Alangaden, G., & Bardossy, A. C. (2018). The role of environmental contamination in the transmission of nosocomial pathogens and healthcare-associated infections. Current Infectious Disease Reports, 20(6), 12. https://doi.org/10.1007/s11908-018-0620-2
Sun, D., Accavitti, M. A., & Bryers, J. D. (2005). Inhibition of biofilm formation by monoclonal antibodies against Staphylococcus epidermidis RP62A accumulation-associated protein. Clinical and Diagnostic Laboratory Immunology, 12(1), 93–100. https://doi.org/10.1128/CDLI.12.1.93-100.2005
Sun, X., Hill, P., Liu, J., Qian, J., Ma, Y., & Zhou, S. (2021). Marine-source Quorum Quenching enzyme YtnP to improve hygiene quality in dental units. Marine Drugs, 19(4), 225. https://doi.org/10.3390/md19040225
Szymańska, M., Karakulska, J., Sobolewski, P., Kowalska, U., Grygorcewicz, B., Böttcher, D., Bornscheuer, U. T., & Drozd, R. (2020). Glycoside hydrolase (PelAh) immobilization prevents Pseudomonas aeruginosa biofilm formation on cellulose-based wound dressing. Carbohydrate Polymers, 246, 116625. https://doi.org/10.1016/j.carbpol.2020.116625
Taylor, P. K., Kessel, A. T. M. V., Colavita, A., Hancock, R. E. W., & Mah, T.-F. (2017). A novel small RNA is important for biofilm formation and pathogenicity in Pseudomonas aeruginosa. PLOS ONE, 12(8), e0182582. https://doi.org/10.1371/journal.pone.0182582
Tenke, P., Mezei, T., Bőde, I., & Köves, B. (2017). Catheter-associated urinary tract infections. European Urology Supplements, 16(4), 138–143. https://doi.org/10.1016/j.eursup.2016.10.001
Thomason, M. K., Fontaine, F., De Lay, N., & Storz, G. (2012). A small RNA that regulates motility and biofilm formation in response to changes in nutrient availability in Escherichia coli. Molecular Microbiology, 84(1), 17–35. https://doi.org/10.1111/j.1365-2958.2012.07965.x
Valentini, M., & Filloux, A. (2016). Biofilms and Cyclic di-GMP (c-di-GMP) Signaling: Lessons from Pseudomonas aeruginosa and other bacteria *. Journal of Biological Chemistry, 291(24), 12547–12555. https://doi.org/10.1074/jbc.R115.711507
Vogel, J., Wakker-Havinga, M., Setroikromo, R., & Quax, W. J. (2020). Immobilized acylase PvdQ reduces Pseudomonas aeruginosa biofilm formation on PDMS silicone. Frontiers in Chemistry, 8, 54. https://doi.org/10.3389/fchem.2020.00054
Vukotic, G., Obradovic, M., Novovic, K., Di Luca, M., Jovcic, B., Fira, D., Neve, H., Kojic, M., & McAuliffe, O. (2020). Characterization, antibiofilm, and depolymerizing activity of two phages active on carbapenem-resistant Acinetobacter baumannii. Frontiers in Medicine, 7, 426. https://doi.org/10.3389/fmed.2020.00426
Wang, D., Chen, H., Li, J., Li, T., Ren, L., Liu, J., & Shen, Y. (2022). Screening and validation of quorum quenching enzyme PF2571 from Pseudomonas fluorescens strain PF08 to inhibit the spoilage of red sea bream filets. International Journal of Food Microbiology, 362, 109476. https://doi.org/10.1016/j.ijfoodmicro.2021.109476
Wang, D., Cui, F., Xi, L., Tan, X., Li, J., & Li, T. (2023). Preparation of a multifunctional non-stick tamarind polysaccharide-polyvinyl alcohol hydrogel immobilized with a Quorum Quenching enzyme for maintaining fish freshness. Carbohydrate Polymers, 302, 120382. https://doi.org/10.1016/j.carbpol.2022.120382
Wang, Z., Vanbever, R., Lorent, J. H., Solis, J., Knoop, C., & Van Bambeke, F. (2024). Repurposing DNase I and alginate lyase to degrade the biofilm matrix of dual-species biofilms of Staphylococcus aureus and Pseudomonas aeruginosa grown in artificial sputum medium: In-vitro assessment of their activity in combination with broad-spectrum antibiotics. Journal of Cystic Fibrosis. https://doi.org/10.1016/j.jcf.2024.02.012
Winzer, K., Falconer, C., Garber, N. C., Diggle, S. P., Camara, M., & Williams, P. (2000). The Pseudomonas aeruginosa lectins PA-IL and PA-IIL are controlled by Quorum Sensing and by RpoS. Journal of Bacteriology, 182(22), 6401–6411. https://doi.org/10.1128/jb.182.22.6401-6411.2000
Wolcott, R. D., Hanson, J. D., Rees, E. J., Koenig, L. D., Phillips, C. D., Wolcott, R. A., Cox, S. B., & White, J. S. (2016). Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair and Regeneration, 24(1), 163–174. https://doi.org/10.1111/wrr.12370
Wu, H., Moser, C., Wang, H.-Z., Høiby, N., & Song, Z.-J. (2015). Strategies for combating bacterial biofilm infections. International Journal of Oral Science, 7(1), 1–7. https://doi.org/10.1038/ijos.2014.65
Ye, J., Shao, C., Zhang, X., Guo, X., Gao, P., Cen, Y., Ma, S., & Liu, Y. (2017). Effects of DNase I coating of titanium on bacteria adhesion and biofilm formation. Materials Science and Engineering: C, 78, 738–747. https://doi.org/10.1016/j.msec.2017.04.078
Zhang, Y., Liu, X., Wen, H., Cheng, Z., Zhang, Y., Zhang, H., Mi, Z., & Fan, X. (2023). Anti-biofilm enzymes-assisted antibiotic therapy against burn wound infection by Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy, 67(7), e00307-23. https://doi.org/10.1128/aac.00307-23
Zhu, B., Liu, Y., Lin, L., Huang, X., Zhang, Y., Zheng, J., & Jin, X. (2019). Characteristics of infectious keratitis in bandage contact lens wear patients. Eye & Contact Lens: Science & Clinical Practice, 45(6), 356–359. https://doi.org/10.1097/ICL.0000000000000593
Zizovic, I., Senerovic, L., Moric, I., Adamovic, T., Jovanovic, M., Krusic, M. K., Misic, D., Stojanovic, D., & Milovanovic, S. (2018). Utilization of supercritical carbon dioxide in fabrication of cellulose acetate films with anti-biofilm effects against Pseudomonas aeruginosa and Staphylococcus aureus. The Journal of Supercritical Fluids, 140, 11–20. https://doi.org/10.1016/j.supflu.2018.05.025