1. Introduction
The preservation of cultural heritage materials presents a complex and multidisciplinary challenge that requires a careful balance between effective microbial control and the long-term safety and integrity of historical substrates (Cappitelli et al. 2025). Traditionally, synthetic biocides have played a central role in the remediation and prevention of biodeterioration in archival documents, artworks, and architectural heritage. However, growing concerns regarding their toxicity, environmental persistence, and potential interactions with fragile organic materials have driven the search for safer and more sustainable alternatives (Cirone et al. 2023; Doni et al. 2024).
Essential oils (EOs), volatile and chemically diverse plant-derived compounds, have emerged as promising bioactive agents in the field of heritage conservation (Diaz-Alonso et al. 2021; Tomić et al. 2023a). Their broad-spectrum antimicrobial, antifungal, and insect-repellent properties are well-documented across various industries, including food preservation, medicine, and agriculture (Micić et al. 2021; Catani et al. 2022; Mounira, 2023; Tomić et al. 2023b). In the context of cultural heritage, EOs offer the additional advantage of biodegradability and lower ecotoxicological impact compared to conventional chemical biocides. The presence of terpenes, phenols, aldehydes, and alcohols in their complex composition makes essential oils highly effective biocidal agents, capable of targeting resistant microorganisms typically found on paper, textile, wooden, stone, and multi-material artifacts (Tanasa et al. 2024).
Despite their potential, the integration of EOs into conservation practice remains limited by several challenges, including their volatility, sensitivity to light and temperature, and the risk of material interactions or aesthetic alterations (Sharmeen Jugreet et al. 2020; Cirone et al. 2023). Moreover, the absence of standardized application methods, clear dosage guidelines, and long-term impact evaluations limits their broader acceptance in professional conservation practice
This review provides a comprehensive overview of the current strategies employed for the application of essential oils in the conservation of cultural heritage materials. It discusses direct and vapor-phase methods, innovative delivery systems such as hydrogels, encapsulated formulations, and emulsions, and critically evaluates their efficacy, limitations, and compatibility with sensitive substrates. The manuscript also identifies key methodological challenges and research gaps that must be addressed to advance the responsible and effective use of essential oils in both preventive and curative conservation settings.
2. Biodegradation of archival papers
The organic nature of paper makes it particularly vulnerable to biodeterioration driven by the metabolic activity of microorganisms. In the case of written cultural heritage, where paper is the predominant substrate, microbiological contamination poses a serious threat to both the physical integrity of materials and the health of individuals handling them. Among the most common biological agents responsible for damage are filamentous fungi, especially genera such as Alternaria, Aspergillus, Cladosporium, Fusarium, and Penicillium, which exhibit high resilience to environmental fluctuations and can colonize paper, parchment, leather, and textiles (Pinheiro et al. 2019; Paolino et al. 2024). While some species cause limited damage, cellulolytic fungi have the capacity to completely degrade cellulose fibers, leading to weakened, fragmented documents and visible discoloration (e.g., foxing). In addition to cellulose, auxiliary materials in paper composition, such as plant- or animal-based adhesives and surface contaminants further serve as nutrient sources for microbial growth (Pinheiro et al. 2019).
Enzymatic degradation of tannins in inks can result in faded text, while metabolic byproducts can create a spectrum of colored stains, whose appearance depends on fungal species, paper composition, microclimate conditions, and microbial interactions. In addition to contaminating surfaces, fungal spores are commonly airborne in archival storage areas, especially in poorly ventilated rooms with significant dust accumulation and elevated moisture levels. This creates a dual hazard: ongoing material degradation and exposure of staff to allergens and potentially toxic mycotoxins (Al Hallak et al. 2023). Consequently, preventive measures, particularly maintaining clean, dry, and climate-controlled storage environments are essential to inhibit microbial growth.
Despite the critical need for intervention, conventional disinfection treatments of contaminated archival materials have traditionally relied on synthetic chemical biocides. While effective, these agents often pose risks to conservators, visitors, and the treated materials themselves, including irreversible aesthetic and chemical alterations (Tomić et al. 2023a; Cirone et al. 2023). In response, researchers and heritage professionals have turned toward bio-based alternatives that are both effective and ecologically safer.
3. Biodegradation of architectural cultural heritage materials
Architectural cultural heritage materials, such as stone, brick, mortar, plaster, and painted surfaces, are continuously exposed to environmental, chemical, and biological stressors that accelerate their deterioration. Among these, microbial colonization represents a significant and often underestimated contributor to material degradation. Biodeterioration of built heritage is commonly initiated by the adhesion of biofilm-forming organisms, including bacteria, algae, lichens, and fungi, which exploit surface irregularities, microcracks, and retained moisture as niches for growth. Once established, these communities alter the physicochemical properties of the substrate through metabolic byproducts, including organic acids, pigments, and exopolysaccharides (Dakal & Cameotra, 2012).
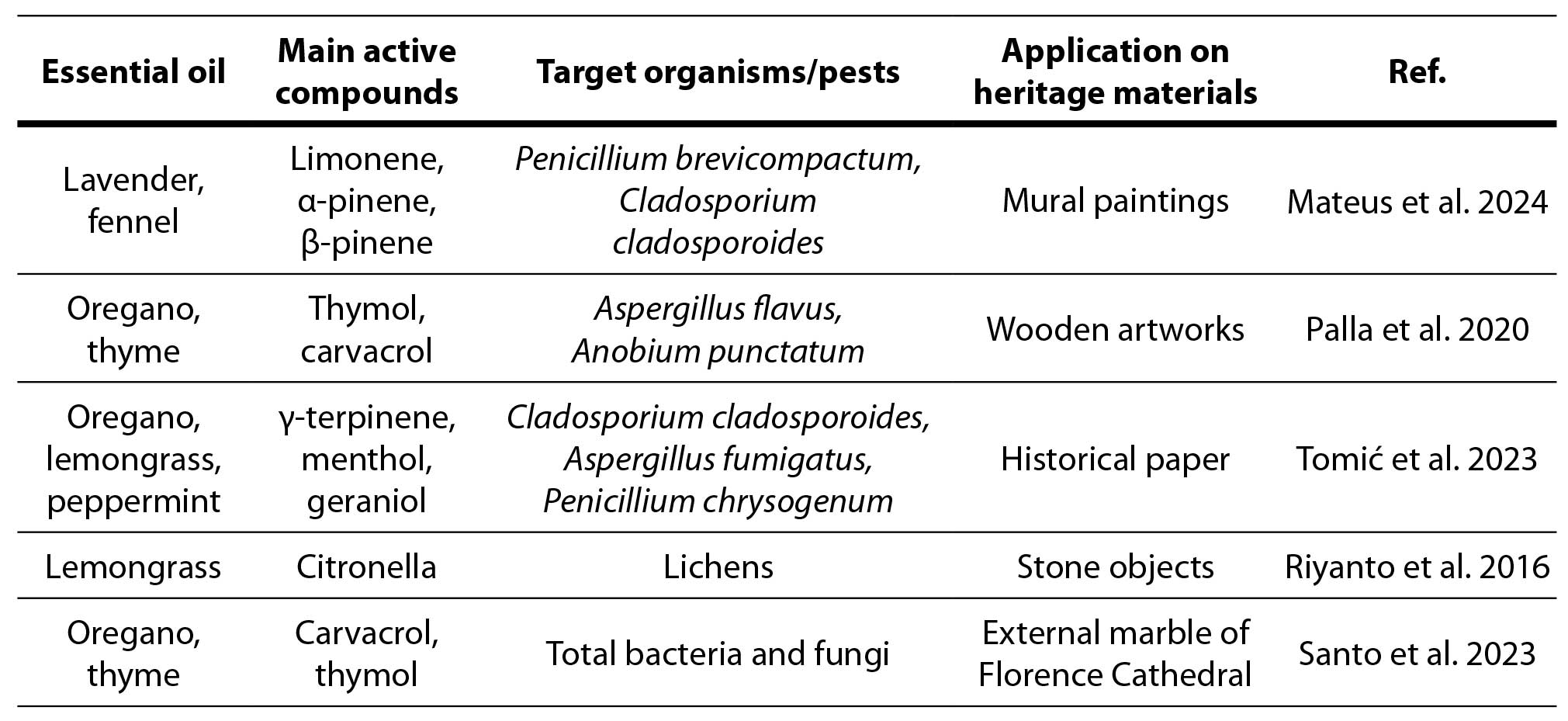
Table 1. Case studies of EOs application on cultural heritage materials
Fungi, especially genera such as Aspergillus, Alternaria, Cladosporium, and Trichoderma, as well as actinobacteria and cyanobacteria, are frequently detected on heritage building surfaces. Their enzymatic activity can lead to the dissolution of binding agents, increased porosity, discoloration, and mechanical weakening of materials. In limestone and marble, organic acid production can result in surface pitting and mineral leaching, while in porous substrates like sandstone, gypsum, or stucco, microbial activity can facilitate salt crystallization and hydration cycles, amplifying physical damage over time (Dakal & Cameotra, 2012; Gadd et al. 2024). Moreover, lichens and mosses contribute to biodeterioration through both biochemical attack and mechanical anchoring, which may cause scaling and detachment of surface layers (Cozzolino et al. 2022).
The visual manifestations of microbial colonization, ranging from dark bio-patina, green or black crusts, and efflorescence to pigment fading and roughened textures, pose not only aesthetic concerns but also reflect deep material compromise. In historic monuments, frescoes, and sculptures, the presence of microbial consortia can lead to irreversible loss of artistic detail and structural cohesion (Vidaković et al. 2013; Gaylarde, 2020).
In light of these challenges, interest has grown in the use of essential oils (EOs) as safer, biodegradable alternatives for microbial control on architectural heritage materials. Their integration into conservation strategies must account for specific material compatibilities, delivery methods, and exposure conditions to ensure efficacy without compromising heritage value.
4. Mechanisms of action of essential oils in the conservation of cultural heritage materials
The application of essential oils (EOs) in the field of cultural heritage conservation has gained significant attention as an environmentally friendly alternative to conventional chemical treatments. Their efficacy is primarily attributed to the diverse biological activities of their volatile constituents, which include antimicrobial, antifungal, insecticidal, and antioxidant properties. These mechanisms are particularly valuable for the preventive and curative conservation of organic and inorganic heritage substrates, such as paper, textiles, wood, stone, and mural paintings.
The primary mechanism by which EOs exert antimicrobial and antifungal effects is through disruption of microbial cell membranes. Most EO constituents are lipophilic in nature, allowing them to penetrate the lipid bilayer of microbial membranes, causing increased permeability, leakage of cellular contents, and ultimately cell lysis (Bakkali et al. 2008). Compounds such as thymol, carvacrol, eugenol, and citral have been shown to interfere with microbial enzymatic systems and nucleic acid synthesis, thereby inhibiting cell metabolism and replication (Burt, 2004; Dorman & Deans, 2000). This activity is particularly relevant for the inhibition of biodeteriogenic microorganisms such as Aspergillus, Penicillium, Cladosporium, and Bacillus species commonly found on heritage materials.
Several essential oils demonstrate repellent or toxic effects against insect pests that pose a threat to cultural heritage objects. For instance, lavender, clove, peppermint, and citronella oils have been reported to deter insects such as silverfish (Lepisma saccharina), woodworms, and carpet beetles (Broda, 2020). The mode of action involves interference with the insect nervous system, particularly via the inhibition of acetylcholinesterase or disruption of the octopaminergic signaling pathway, leading to paralysis or death (Isman, 2000).
EOs also contribute to the stabilization of heritage materials through their antioxidant properties. Phenolic constituents such as eugenol, rosmarinic acid, and carnosic acid can neutralize reactive oxygen species (ROS), thereby reducing oxidative degradation processes in sensitive organic substrates such as paper, textiles, or natural dyes (Miguel, 2010). This function may be especially beneficial in mitigating damage from environmental pollutants and photooxidation.
The ability of EOs to penetrate and destabilize microbial biofilms is an added advantage in conservation practices, as biofilms provide a protective matrix that enhances microbial resistance to traditional biocides. EO constituents have been shown to disrupt quorum sensing and inhibit the formation and maintenance of biofilms, leading to a reduction in microbial colonization on stone, frescoes, and other porous materials (Nazzaro et al. 2013). Chosen case studies of the application of EOs in conservation of cultural heritage is given in Table 1.
EOs can be applied in various forms, including vapor-phase fumigation, microemulsions, or in encapsulated systems for controlled release. However, compatibility with substrates must be carefully assessed through preliminary tests, as high concentrations or prolonged exposure may cause color changes, surface alterations, or residual odors. It is essential to optimize concentration, exposure time, and application technique for each specific material.
In the following text the most frequently and novel methods and delivery systems of essential oils are explained in details.
5. Direct application techniques
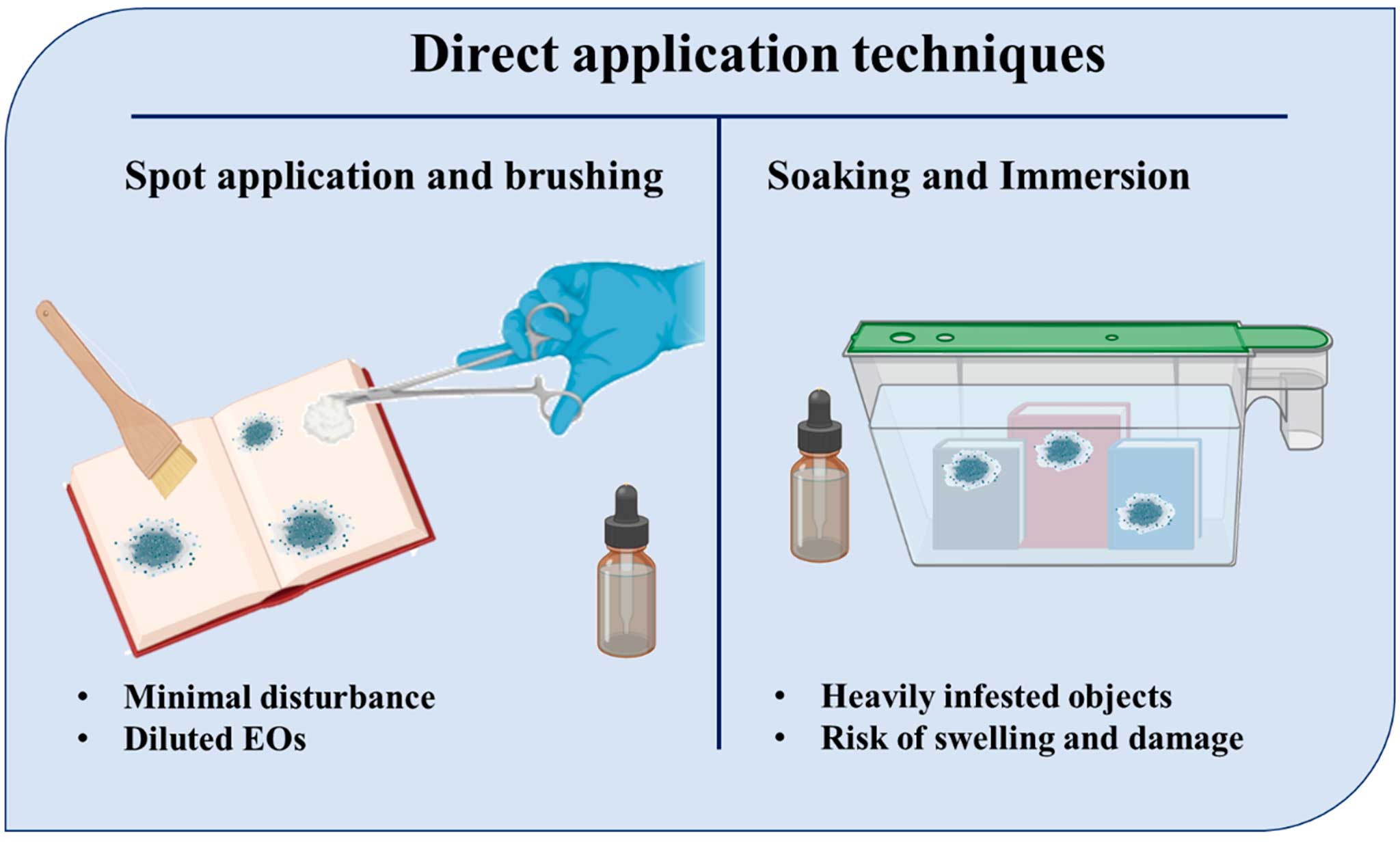
Direct application remains one of the most straightforward and accessible methods for utilizing EOs in heritage conservation. This approach primarily involves either spot application through brushing or swabbing, or soaking for more thorough penetration in severely deteriorated objects (Russo & Palla, 2023). These methods (Figure 1) are particularly common for treating organic substrates such as parchment, textiles, and wood, materials known for their susceptibility to microbial colonization and insect infestation (Palla et al. 2020).
Spot application using brushes, cotton swabs, or microfiber pads enables conservators to target specific contaminated areas with minimal disturbance to surrounding surfaces. Essential oils are typically diluted in ethanol or other compatible solvents to improve control and avoid excessive residue (Antonelli et al. 2024). Essential oils such as clove, lemongrass, oregano, rosemary, peppermint, and eucalyptus have been successfully applied using this method to parchment, book bindings, historical paper and textile fragments where localized fungal activity is evident (Pop et al. 2022). The advantages of this method lie in its simplicity, low material cost, and manual precision, which allows the conservator to control the amount and location of application. However, the effectiveness of this technique is often compromised by the volatile nature of essential oils, which can lead to rapid evaporation before the full antimicrobial or antifungal action is achieved (Antonelli et al. 2020). Additionally, uneven penetration can occur, especially in layered or dense materials like tanned leather or compact wood pulp, potentially resulting in insufficient disinfection beneath the surface layer (Reale et al. 2024).
Figure 1. Direct essen tial oil(s) application techniques in conservation procedures
In cases of severe microbial infestation, such as mold colonization of wooden artifacts or contaminated textiles, soaking or immersion in EO solutions may be considered. This method is generally employed in a controlled environment, with immersion times carefully adjusted based on the object’s porosity and fragility (Gadd et al. 2024). Studies have demonstrated the antimicrobial efficacy of full immersion treatments, particularly when using EO blends rich in phenolic compounds such as carvacrol and eugenol (Khwaza & Aderibigbe, 2025). Nevertheless, this technique comes with substantial risks. Prolonged exposure to EO-rich solvents can saturate delicate organic materials, leading to potential swelling, softening or pigment bleeding, especially in dyed textiles or painted wooden surfaces (Negi, 2025). It is therefore crucial that any immersion process be preceded by thorough material testing and risk assessment. In conservation practice, soaking is rarely a first-line treatment. It is more appropriately applied as a last resort when the biological deterioration is extensive and threatens the structural integrity of the artifact (Artesani et al. 2020).
6. Vapor-phase applications and fumigation techniques
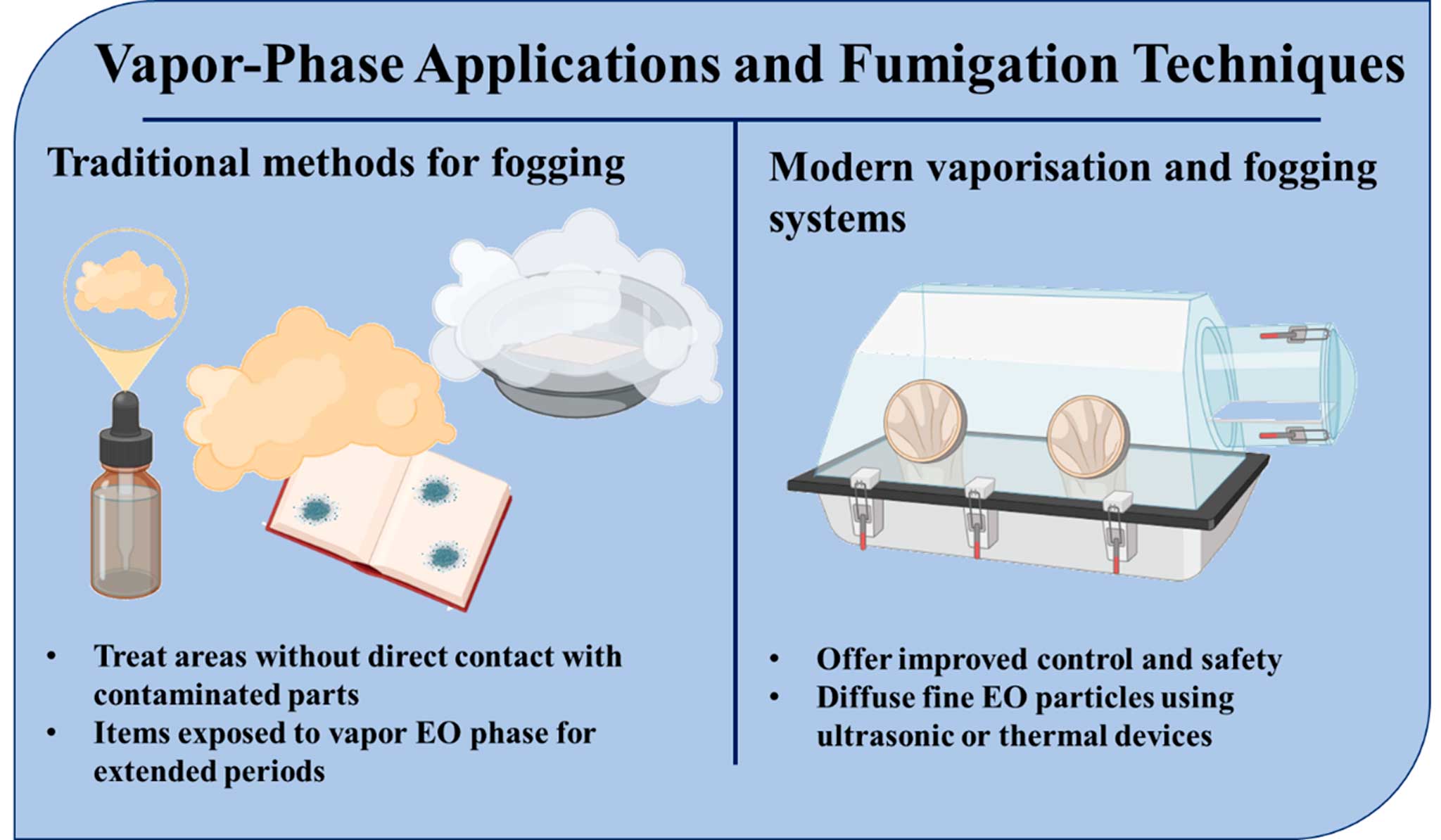
The vapor-phase application of EOs represents one of the oldest and most passive biocidal strategies (Figure 2) in the conservation of cultural heritage materials, particularly archival documents and stored collections. This method leverages the volatility of EOs to distribute antimicrobial compounds in a closed environment, enabling non-invasive disinfection of sensitive and often inaccessible materials (Mateus et al., 2024).
Thymol fumigation, pioneered in the 1970s, remains a common protocol in document conservation, especially for paper-based materials vulnerable to fungal and insect attack. Thymol, a monoterpenoid phenol extracted primarily from Thymus vulgaris, was favored due to its broad-spectrum fungicidal activity and relatively benign interactions with cellulose-based supports (Reale et al., 2024). These early methods involved the use of sealed chambers or steel cabinets where documents were exposed to thymol vapor for extended periods. While cost-effective and relatively easy to implement, the lack of dosage control and standardized exposure times often led to uncertain biocidal outcomes, and prolonged exposure raised concerns about residual deposition and material discoloration, particularly in inks and varnished papers (Mateus et al. 2024).
Recent advancements have led to the development of commercial EO vaporization systems, which offer improved control and safety. A notable example is BACTIGAS®, a commercial aerosol product that releases tea tree oil in fine mist form. Originally developed for HVAC sanitization, it has been adapted for preventive mold control in large museum storage areas (Reale et al. 2024). Another emerging approach is essential oil fogging, which diffuses fine EO particles using ultrasonic or thermal devices. This technique allows for uniform distribution in enclosed environments, such as display cases, storage vaults, or transport crates, and has shown promise in reducing bacterial and fungal loads on exposed and semi-enclosed surfaces (Bastholm et al. 2022).
The primary advantage of vapor-phase EO application lies in its ability to treat difficult to reach areas without requiring direct contact with the object. This makes it particularly useful for the preservation of large, fragile, or densely stored collections, where brushing or spraying might pose a mechanical risk. Furthermore, the non-invasive nature of vapor diffusion helps protect the structural integrity of delicate materials such as aged parchment, brittle paper, and bound manuscripts (Palla et al. 2020). However, vapor-phase treatments are not without limitations. The lack of surface contact may result in insufficient microbial penetration, particularly for deeply embedded infestations. Additionally, volatile dispersion leads to uneven concentration gradients, which can cause inconsistent efficacy across different materials or object geometries (Soldano et al. 2020). Certain EOs may also leave residual aromatic compounds, raising concerns about olfactory contamination or unwanted material interaction (Miri et al. 2025). Thus, while EO vaporization remains a valuable component of preventive conservation strategies, it should be applied in controlled settings and ideally combined with targeted surface treatments for comprehensive biocidal efficacy.
Figure 2. Vapor-phase applications
Spraying and atomization represent one of the most practical and scalable approaches to applying EOs in the field of cultural heritage conservation. These techniques (Figure 3) are especially useful for large surface areas, including vertical walls, frescoed plaster, stone monuments, and display environments where microbial control is required but physical contact must be minimized (Bosh-Roig et al. 2015).
Spraying methods can range from low-tech manual spray bottles to pressurized atomizers and professional-grade backpack sprayers commonly used in outdoor archaeological settings. Essential oils such as oregano, thyme, tea tree, and citronella are among the most frequently used in spray applications. These EOs have well-documented antifungal and antibacterial properties and are known to be effective against pathogens commonly found on stone, stucco, mural surfaces, and wall paintings (Palla et al. 2020; Sanchis et al. 2023).
6.1 Application use cases
In view of surface cleaning treatments, diluted EO solutions are sprayed directly onto biologically contaminated surfaces such as limestone façades, stucco, or frescoes. After a dwell time, mechanical removal of biofilms may follow. Such techniques have proven effective against green algae, lichens, and cyanobacteria, especially when using EO emulsions stabilized with surfactants or clays (Sasso et al. 2013; Gagliano Candela et al. 2019). For preventive coatings, EO sprays are also used to create a temporary antimicrobial barrier on museum walls, storage containers, and showcases. When used intermittently, these coatings can deter microbial growth in climate-controlled spaces without the need for invasive cleaning (Sala-Luis et al. 2024).
Figure 3. Spraying and atomization techniques in EO application
While spraying is advantageous for ease of use, broad coverage, and non-contact delivery, there are important material compatibility issues to consider. Because essential oils are lipophilic, they can penetrate porous substrates, leaving visible residues, especially on light-colored or sensitive surfaces such as marble, lime plaster, or painted layers (Cennamo et al. 2023). Some oils—particularly those rich in aldehydes like cinnamon or phenols like thymol—can cause chromatic alterations or a slight yellowing effect, especially if not properly diluted (Genova et al. 2023). Moreover, hydrophobic staining and prolonged aromatic residue may pose a challenge in enclosed environments such as museum displays. As a result, post-application monitoring and material testing are strongly recommended prior to full-scale implementation (Cennamo et al. 2023). Despite these caveats, spraying remains a versatile and efficient delivery method, especially when paired with formulations like microemulsions or encapsulated EOs, which reduce volatility and enhance penetration control.
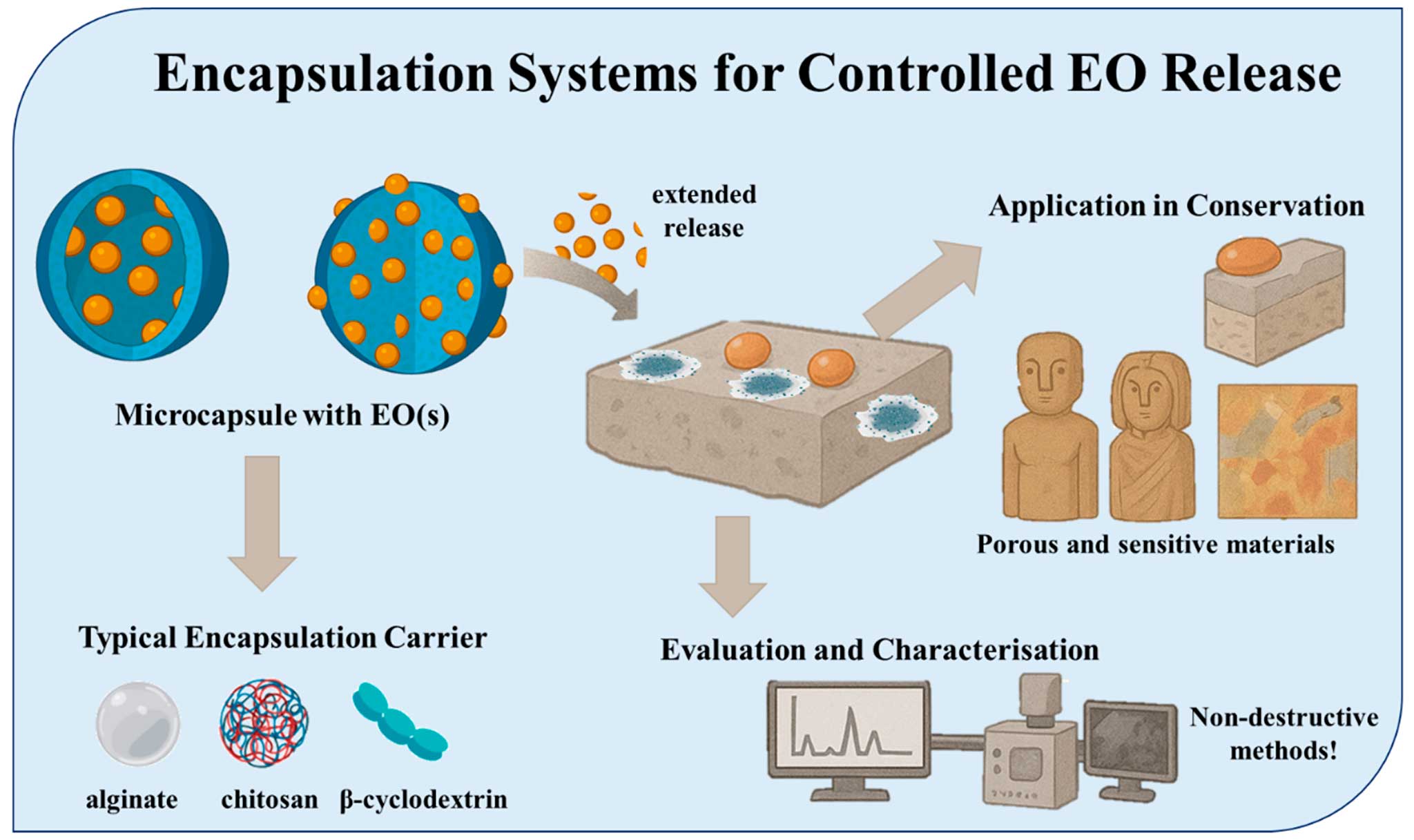
7. Encapsulation systems for controlled EO release
Encapsulation technologies are at the forefront of efforts to enhance the stability, efficacy, and precision of EO applications in heritage conservation (Figure 4). By embedding volatile EO compounds within protective matrices, encapsulation allows for sustained release, reduced evaporation, and better material compatibility, essential features when dealing with sensitive and valuable artefacts (Ayyaril et al. 2023). Encapsulation methods typically rely on biopolymeric carriers, such as alginate (a polysaccharide derived from brown algae), chitosan (from chitin, commonly found in crustacean shells), and β-Cyclodextrin (β-CD) (a cyclic oligosaccharide with a hydrophobic core). The polymers form microcapsules or gel beads that can house EOs and gradually release their active compounds in response to environmental conditions such as humidity, temperature, or pH (Vadrucci, 2025). In addition to their role as structural carriers, it is important to note that some biopolymers used in encapsulation, especially chitosan, exhibit notable antimicrobial properties. Chitosan’s cationic nature enables it to interact with negatively charged microbial cell membranes, disrupting their permeability, leading to leakage of cellular contents and microbial death. This activity has been documented against a wide spectrum of bacteria and fungi (Goy et al. 2009). Similarly, while alginate is generally considered biologically inert, certain formulations can influence microbial adhesion, biofilm formation, or diffusion properties, indirectly affecting microbial viability (Szekalska et al. 2016; Muxika et al. 2017). These effects may act synergistically or additively with the encapsulated essential oils, potentially complicating the attribution of antimicrobial activity solely to the EO component. Therefore, inclusion of proper controls—such as unloaded carrier systems—is essential to accurately evaluate the specific contribution of essential oils versus the carrier matrix in conservation studies. Nevertheless, this method significantly increases the residence time of EOs on treated surfaces, improving their biocidal effectiveness without the need for repeated application.
Figure 4. Encapsulation systems for Controlled EO release
Encapsulated EO systems have shown particular promise on porous and sensitive materials such as stone, frescoes, wooden sculptures, and wall paintings, where direct application of liquid EOs may lead to surface saturation or unwanted chromatic effects (Mateus et al. 2024). In experimental conservation treatments, alginate or chitosan-EO beads have been strategically placed in test zones or mock-ups to evaluate controlled microbial inhibition over time. These systems have been shown to limit fungal colonization, especially in outdoor conditions where environmental fluctuations typically diminish EO efficacy (Palla et al. 2020). Moreover, encapsulation reduces the risk of aesthetic alteration, as the gradual release minimizes oil pooling or staining often observed with pure EO applications. The performance of encapsulated EO systems is routinely evaluated through analytical instrumentation such as Gas Chromatography–Mass Spectrometry (GC-MS) and scanning electron microscopy (SEM). GC-MS is employed to monitor the retention and release profile of EO components over time. This allows researchers to determine how much of the original oil remains after encapsulation and how steadily it diffuses into the environment (Filatov et al. 2023). SEM is used to examine any morphological changes on treated surfaces, ensuring that no microstructural degradation occurs during or after treatment (Ural, 2021). Some studies have also introduced in vitro models for testing encapsulated EO beads against specific biofilms or fungal species, offering a highly controlled, reproducible evaluation method prior to field application (Cattò & Cappitelli, 2019).
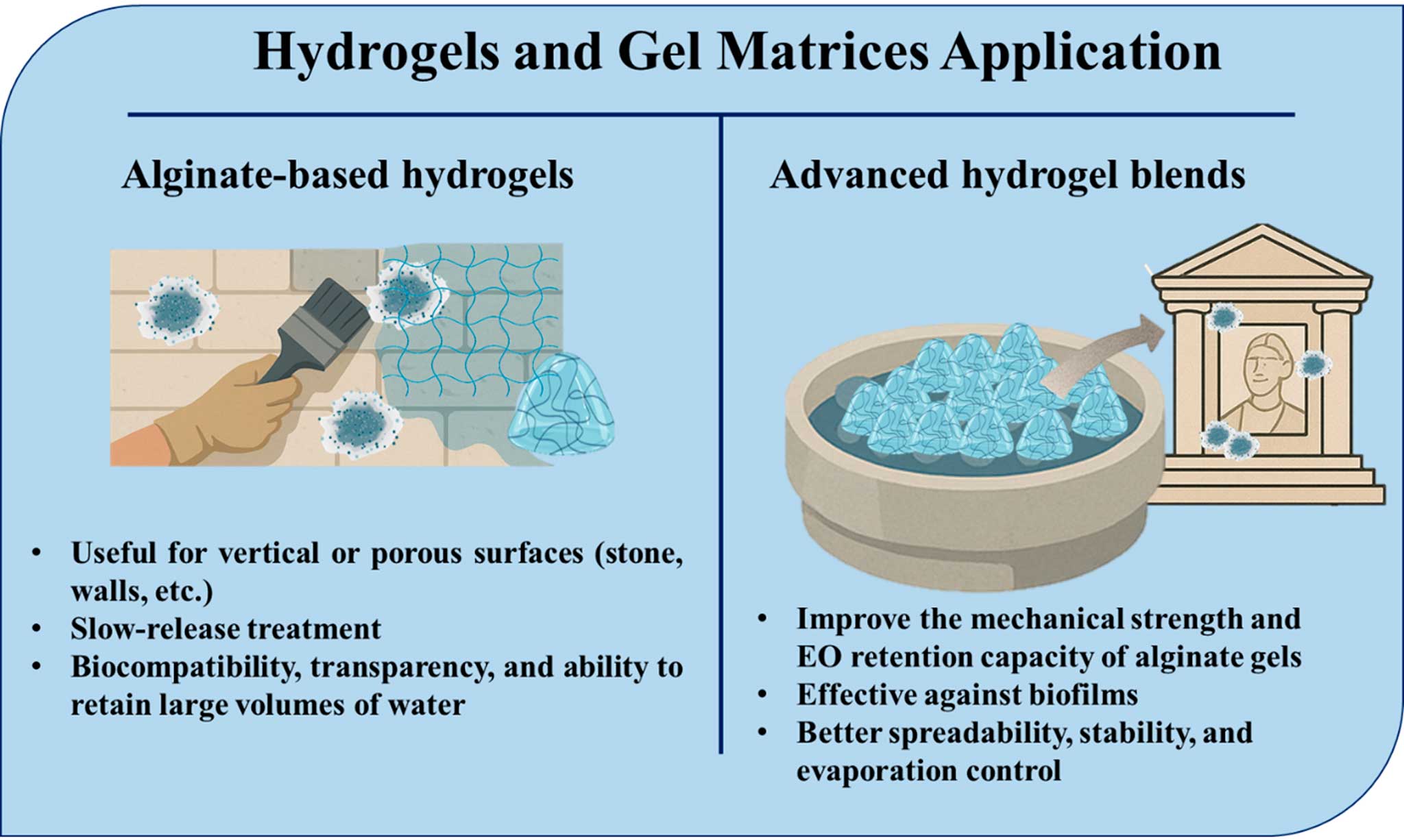
8. Hydrogels and gel matrices
In the ongoing quest for sustainable and material-safe delivery systems for EOs, hydrogels have emerged as a highly adaptable and non-invasive platform, particularly for treating vertical, irregular, or porous heritage surfaces (Figure 5). These semi-solid, water-retentive matrices allow for localized, prolonged EO release while minimizing risks of mechanical or chemical damage to artworks (Chelu, 2024).
Alginate, a naturally derived biopolymer from brown algae, has gained popularity in cultural heritage conservation due to its biocompatibility, transparency, and ability to retain large volumes of water. When cross-linked (typically with calcium chloride), it forms a flexible gel network capable of holding EOs such as thyme, oregano, and cinnamon in suspension. These systems are particularly suitable for vertical surfaces (e.g., stone façades, wall paintings, and mosaics), where traditional EO liquid application would drip or evaporate too rapidly (Chaban et al. 2020). The slow-release profile of EOs from alginate gels enables extended contact with microbial colonies, enhancing their antimicrobial performance.
Figure 5. Hydrogels and gel matrices application
To improve the mechanical strength and EO retention capacity of alginate gels, researchers have developed hybrid systems. For example, incorporating psyllium husk, a natural swelling agent, increases alginate gel viscosity and enhances surface adhesion on textured substrates. PVA (polyvinyl alcohol)-based hydrogels are synthetic yet biocompatible, offering improved gel integrity and reduced oil volatilization. Gellan gum matrices are known for their strong gelation and film-forming properties, gellan-based gels offer high EO encapsulation efficiency and are effective against mixed-species biofilms on stone and painted surfaces (Shaikh et al. 2021; Liang et al. 2024). These blends offer better spreadability, stability, and evaporation control, making them ideal for outdoor applications where temperature and humidity fluctuate. Overall, EO-loaded hydrogels are among the most promising eco-compatible options for conservation professionals seeking targeted, reversible, and minimally invasive treatments.
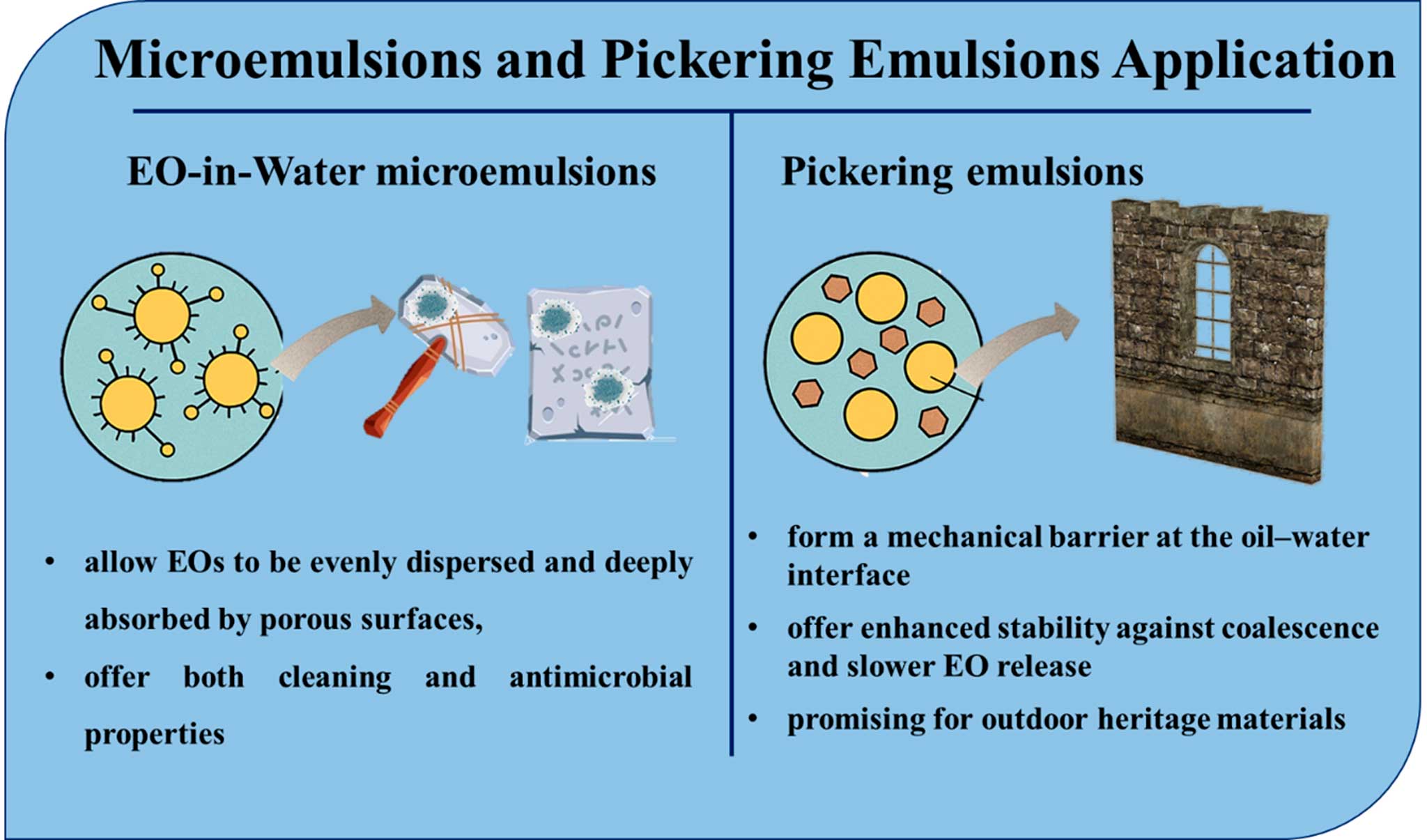
9. Microemulsions and pickering emulsions
As the conservation field seeks increasingly refined and eco-compatible delivery methods for EOs, emulsion-based systems, particularly microemulsions and Pickering emulsions, have emerged as innovative tools (Figure 6). These formulations aim to stabilize volatile oils in water-based environments, ensuring effective application on delicate materials without excessive residue or uncontrolled diffusion (Lucia & Guzmán, 2021; Cahyana et al. 2022)
Microemulsions are clear, thermodynamically stable dispersions of oil and water, typically stabilized with non-ionic surfactants. When used in cultural heritage, they allow EOs to be evenly dispersed and deeply absorbed by porous surfaces, offering both cleaning and antimicrobial properties (Tartaro et al. 2020). Such systems are especially effective on metallic surfaces, where EOs act as both antifungal agents and corrosion inhibitors, and ceramics and stone, where surface hydrophobicity must be minimized. The low viscosity of microemulsions contributes to their exceptional wetting ability, improving penetration into crevices, inscriptions, or relief features (Cui et al., 2021). Unlike conventional emulsions that rely on surfactants, Pickering emulsions are stabilized by solid particles, such as natural clays (e.g., bentonite, sepiolite). These form a mechanical barrier at the oil–water interface, offering enhanced stability against coalescence and slower EO release (Binks, 2002). Pickering emulsions are particularly promising for outdoor heritage materials, including limestone, ceramic fragments, and architectural stone, as they retain the EO longer on surfaces exposed to rain and UV light, prevent oil pooling or streaking, and can be customized by altering clay type and concentration (Cahyana et al., 2022).
Figure 6. Microemulsions and pickering emulsions
Advantages of both micro- and pickering emulsions include superior surface wetting, even on rough or porous substrates, low EO concentration required, minimizing the risk of staining, and ease of application via spraying or brushing, especially on vertical or irregular surfaces. However, some challenges persist. Emulsion stability over time can be affected by temperature, pH, and origin of EO, necessitating on-demand preparation or cold storage. Certain EOs, especially those rich in aldehydes (e.g., cinnamaldehyde), can still lead to subtle color changes on light or unsealed surfaces, especially after repeated applications (de Carvalho-Guimarães et al. 2022). The physical removal of clay residues in Pickering emulsions may require gentle rinsing, which can pose risks for water-sensitive substrates (Wang & Wang, 2016; Cho et al. 2018). Nevertheless, these systems offer one of the most balanced solutions for EO application, combining efficacy, control, and material compatibility and are rapidly gaining traction in both preventive and curative conservation protocols.
10. Methodological challenges and research gaps
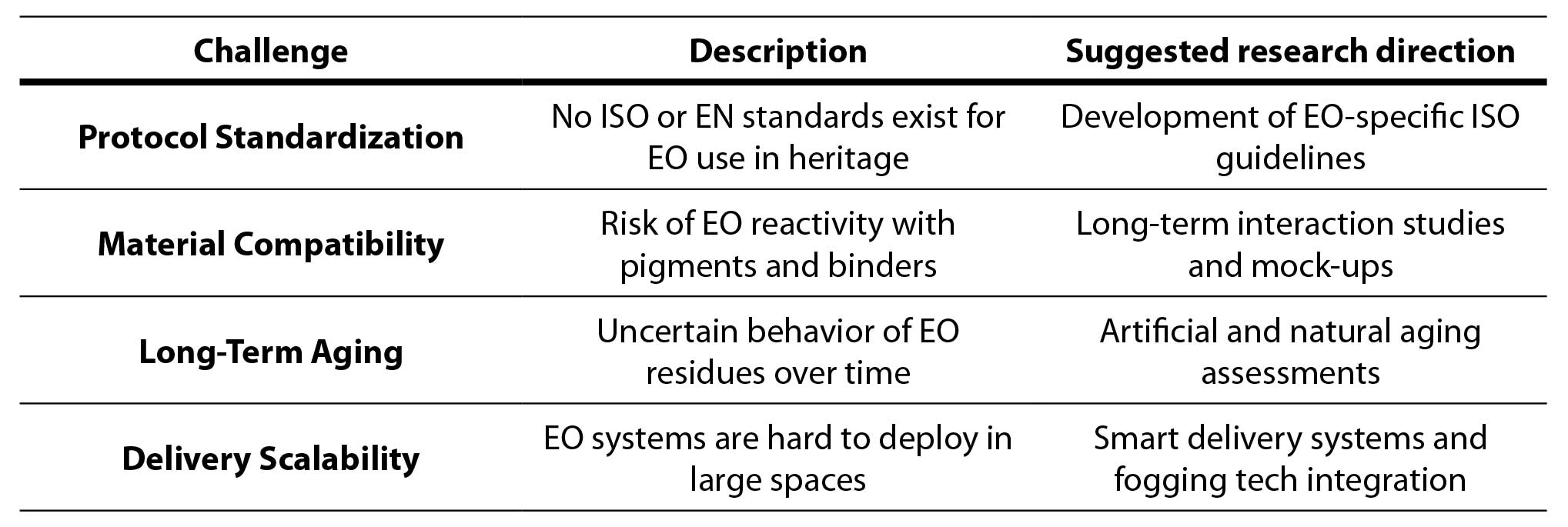
Although essential oils (EOs) have demonstrated promising antimicrobial effects in laboratory conditions, their practical implementation in cultural heritage conservation is still constrained by several specific methodological limitations (Table 2). One of the primary obstacles is the absence of comparative studies that assess the efficacy of EOs on different heritage materials, such as paper, stone, textiles, and wood, under harmonized experimental conditions. Without such data, it is difficult to define material-specific protocols or anticipate unwanted interactions (Russo & Palla, 2023).
Moreover, most current findings are derived from in vitro models or short-term treatments, while real-life conservation environments involve complex and fluctuating factors such as humidity, light, and pollution. This highlights the need for validated in situ testing models and long-term monitoring protocols that would allow assessment of EO persistence, potential microbial recolonization, and overall treatment stability. Another unresolved issue is the limited understanding of chemical interactions between EO components and the sensitive materials often present in historical objects—particularly dyes, pigments, binders, and adhesives. Potential alterations in color, gloss, or texture due to prolonged EO exposure remain insufficiently explored and demand both accelerated and natural aging studies (Singh & Pulikkal, 2022).
A further complication is the lack of standardized microbiological assessment methods tailored to heritage settings. Quantitative evaluation of EO efficacy is often inconsistent, as techniques such as ATP bioluminescence, microbial culturing, or molecular tools like qPCR are rarely adapted or validated for fragile or irreplaceable materials. In addition, the principle of reversibility, which is a cornerstone of modern conservation ethics, is not adequately addressed in the existing literature. There is scarce data on whether EO-based treatments can be safely removed or reapplied over time without causing cumulative material degradation.
Table 2. Summary of research gaps
To overcome these barriers, future innovations should aim toward the development of sensor-triggered delivery systems capable of responding to environmental cues such as increased humidity or microbial presence. For example, nanotechnology-based encapsulation systems, such as EO-loaded nanogels or electrospun fibers, could provide extended release and deeper substrate penetration, while minimizing adverse effects. Finally, the establishment of internationally recognized standards and conservation-specific guidelines is essential to ensure reproducibility, scalability, and safety. Addressing these research gaps through interdisciplinary collaboration will be crucial in transforming EO-based conservation from an experimental approach into a robust and ethically grounded professional practice (Table 2).
11. Conclusion
The transition from synthetic biocides to EO-based treatments marks a significant step toward safer, more sustainable conservation practices. However, the efficacy of EOs is not solely dependent on their chemical composition but is strongly influenced by the method of application. Whether through vapor-phase diffusion, spraying, hydrogel embedding, microemulsions, or encapsulation systems, each delivery mode brings its own set of advantages and limitations, especially in relation to surface compatibility, persistence, and user control. Despite promising laboratory results and growing field applications, the field still lacks standardized, conservation-specific protocols. There is an urgent need for international harmonization of methodologies, including dosage guidelines, safety profiles, and application procedures that take into account material sensitivity and reversibility. Equally critical is the development of long-term risk assessment tools, including accelerated aging studies, to ensure that EO-based treatments align with core conservation ethics and do not unintentionally compromise artifact integrity over time. Looking ahead, the future of EO application lies in hybrid strategies, integrating nanotechnology, microencapsulation, and smart release systems that can respond to environmental cues such as humidity, microbial presence, or light exposure. Innovations like EO-loaded nanogels, self-renewing protective films, or sensor-activated foggers offer exciting possibilities for museum-wide disinfection, while maintaining the reversibility and selectivity demanded by conservation science. Ultimately, EOs are not a universal solution, but rather a toolkit of biocompatible, bioactive compounds whose full potential can only be realized through rigorous interdisciplinary collaboration between chemists, microbiologists, conservation scientists, and heritage professionals.
Acknowledgments: This research was funded by the Ministry of Science, Technological Development and Innovation of the Serbia, under the following grant numbers: 451-03-136/2025-03/200134 and 451-03-137/2025-03/200134.
Conflicts of Interest: The authors declare no conflicts of interest.
References:
Al Hallak, M., Verdier, T., Bertron, A., Roques & C., Bially, J.D. (2023). Fungal Contamination of Building Materials and the Aerosolizationof Particles and Toxins in Indoor Air and Their Associated Risk to Health: A Review. Toxins, 15(3), 175.
Antonelli, F., Bartolini, M., Plissonnier, M.-L., Esposito, A., Galotta, G., Ricci, S., Davidde Petriaggi, B., Pedone, C., Di Giovanni, A., Piazza, S., Guerrieri, F., & Romagnoli, M. (2020). Essential Oils as Alternative Biocides for the Preservation of Waterlogged Archaeological Wood. Microorganisms, 8(12), 2015.
Antonelli, F., Iovine, S., Sacco Perasso, C., Macro, N., Gioventù, E., Capasso, F. E., & Bartolini, M. (2024). Essential Oils and Essential Oil-Based Products Compared to Chemical Biocides Against Microbial Patinas on Stone Cultural Heritage. Coatings, 14(12), 1546.
Ayyaril, S.S., Rawas-Qalaji, M. , Shanableh A., Cagliani R., Bhattacharjee, S., Shabib, A.G. & Khan, M.I. (2023). Recent Progress in Micro and Nano-Encapsulation Techniques for Environmental Applications: A Review. Results in Engineering, 18, 101094.
Bakkali, F., Averbeck, S., Averbeck, D., & Idaomar, M. (2008). Biological effects of essential oils – A review. Food and Chemical Toxicology, 46(2), 446–475.
Bastholm, C.J., Madsen, A.M., Andersen, B., Frisvad, J.C. &F Richter, J. (2022). The MysteriousMould Outbreak – A Comprehensive Fungal Colonisation in a Climate-Controlled Museum Repository Challenges the Environmental Guidelines for Heritage Collections. Journal of Cultural Heritage, 55, 78-87.
Ben Miri Y. (2025). Essential Oils: Chemical Composition and Diverse Biological Activities: A Comprehensive Review. Natural Product Communications, 20(1), doi: 10.1177/1934578X241311790.
Binks, B.P. (2002). Particles as Surfactants—Similarities and Differences. Current Opinion in Colloid & Interface Science, 7, 21–41.
Bosch-Roig, P., Lustrato, G., Zanardini, E. & Ranalli, G. (2015). Biocleaning of Cultural Heritage stone surfaces and frescoes: which delivery system can be the most appropriate? Annals of Microbiology, 65, 1227–1241.
Broda, M. (2020). Natural compounds for wood protection against fungi – a review. Molecules, 25(15),, 3538.
Burt, S. (2004). Essential oils: Their antibacterial properties and potential applications in foods—A review. International Journal of Food Microbiology, 94(3), 223–253.
Cahyana, Y., Putri, Y.S.E., Solihah, D.S., Lutfi, F.S., Alqurashi, R.M., & Marta, H. (2022). Pickering Emulsions as Vehicles for Bioactive Compounds from Essential Oils. Molecules, 27(22), 7872.
Cappitelli, F., Catto, C. & Villa, F. (2025). The Control of Cultural Heritage Microbial Deterioration. Microorganisms, 8, 1542.
Catani, L., Grassi, E., di Montanara, A.C., Guidi, L., Sandulli, R., Manachini, B. & Semprucci, F. (2022). Essential Oils and Their Applications in Agriculture and Agricultural Products: A Literature analysis through VOSviewer. Biocatalysis and Agricultural Biotechnology, 45, 102502.
Cattò, C., & Cappitelli, F. (2019). Testing Anti-Biofilm Polymeric Surfaces: Where to Start? International Journal of Molecular Sciences, 20(15), 3794.
Cennamo, P., Scielzo, R., Rippa, M., Trojsi, G., Carfagna, S., & Chianese, E. (2023). UV-C Irradiation and Essential-Oils-Based Product as Tools to Reduce Biodeteriorates on the Wall Paints of the Archeological Site of Baia (Italy). Coatings, 13(6), 1034.
Chaban, A., Deiana, R., & Tornari, V. (2020). Wall Mosaics: A Review of On-Site Non-Invasive Methods, Application Challenges and New Frontiers for Their Study and Preservation. Journal of Imaging, 6(10), 108.
Chelu, M. (2024). Hydrogels with Essential Oils: Recent Advances in Designs and Applications. Gels, 10(10), 636.
Cho Y.-J., Kim D.-M., Song I.-H., Choi J.-Y., Jin S.-W., Kim B.-J., Jeong J.-W., Jang C.-E., Chu K. & Chung C.-M. (2018). An Oligoimide Particle as a Pickering Emulsion Stabilizer. Polymers, 10, 1071.
Cirone, M., Figoli, A., Galiano, F., La Russa, M.F., Macchia, A., Mancuso, R., Ricca, M., Rovella, N., Traverniti, M. & Ruffolo, S.A. (2023). Innovative Methodologies for the Conservation of Cultural Heritage against Biodeterioration: A Review. Coatings, 13(12), 1986.
Cozzolino, A., Adamo, P., Bonanomi, G. & Motti, R. (2022). The Role of Lichens, Mosses, and Vascular Plants in the Biodeterioration of Historic Buildings: A Review. Plants, 11(24), 3429.
Cui, F., Zhao, S., Guan, X., McClements, D.J., Liu, X., Liu, F. & Ngai, T. (2021). Polysaccharide-Based Pickering Emulsions: Formation, Stabilization and Applications. Food Hydrocolloids, 119, 106812.
Dakal, T.C. & Cameotra, S.S. (2012). Microbially Induced Deterioration of Architectural Heritages: Routes and Mechanisms Involved. Environmental Sciences Europe, 24, 36.
de Carvalho-Guimarães, F.B., Correa, K.L., de Souza, T.P., Rodríguez Amado, J.R., Ribeiro-Costa, R.M., & Silva-Júnior, J.O.C. (2022). A Review of Pickering Emulsions: Perspectives and Applications. Pharmaceuticals, 15(11), 1413.
Doni, M., Fierascu, I. & Claudiu Fierascu, R. (2024). Recent Developments in Material Science for the Conservation and Restoration of Historic Artifacts. Applied Sciences, 14(23), 11363.
Dorman, H. J. D., & Deans, S. G. (2000). Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. Journal of Applied Microbiology, 88(2), 308–316.
Díaz-Alonso, J., Bernardos, A., Regidor-Ros, J.L., Martínez-Manez, R. & Bosch-Roig, P. (2021). Innovative Use of Essential Oil Cold Diffusion System for Improving Air Quality on Indoor Cultural Heritage Spaces. International Biodegradationa and Biodeterioration, 162, 105–125.
Filatov, V.A., Ilin, E.A., Kulyak, O.Y. & Kalenikova, E.I. (2023). Development and Validation of a Gas Chromatography-Mass Spectrometry Method for the Analysis of the Novel Plant-Based Substance with Antimicrobial Activity. Antibiotics, 12(10), 1558.
Gadd, G.M., Fomina, M. & Pinzari, F. (2024). Fungal Biodeterioration and Preservation of Cultural Heritage, Artwork and Historical Artifacts: Extremophyily and Adaptation. Microbiology and Molecular Biology Reviews, 88(1), e00200-22.
Gagliano Candela, R., Maggi, F., Lazzara, G., Rosselli, S., & Bruno, M. (2019). The Essential Oil of Thymbra capitata and its Application as A Biocide on Stone and Derived Surfaces. Plants, 8(9), 300.
Gaylarde, C.C. (2020). Influence of Environment on Microbial Colonization of Historic Stone Buildings with Emphasis on Cyanobacteria. Heritage, 3(4), 1469-1482.
Genova, C., Fuentes, E., Favero, G., & Prieto, B. (2023). Evaluation of the Cleaning Effect of Natural-Based Biocides: Application on Different Phototropic Biofilms Colonizing the Same Granite Wall. Coatings, 13(3), 520.
Goy, R. C., de Britto, D., & Assis, O. B. G. (2009). A review of the antimicrobial activity of chitosan. Polímeros: Ciência e Tecnologia, 19(3), 241–247.
Isman, M. B. (2000). Plant essential oils for pest and disease management. Crop Protection, 19(8-10), 603–608.
Khwaza V & Aderibigbe B.A. (2025). Antibacterial Activity of Selected Essential Oil Components and Their Derivatives: A Review. Antibiotics, 14(1), 68.
Liang, X., Zhong, H.-J., Ding, H., Yu, B., Ma, X., Liu, X., Chong, C.-M., & He, J. (2024). Polyvinyl Alcohol (PVA)-Based Hydrogels: Recent Progress in Fabrication, Properties, and Multifunctional Applications. Polymers, 16(19), 2755.
Lucia, A. & Guzman, E. (2021). Emulsions containing essential oils, their components or volatile semiochemicals as promising tools for insect pest and pathogen management. Advanced in Colloid and Interface Science, 287, 102330.
Mateus, D., Costa, F., de Jesus, V., & Malaquias, L. (2024). Biocides Based on Essential Oils for Sustainable Conservation and Restoration of Mural Paintings in Built Cultural Heritage. Sustainability, 16(24), 11223.
Micić, D., Đurović, S., Riabov, P., Tomić, A., Šovljanski, O., Filip, S., Tosti, T., Dojčinović, B., Božović, R., Jovanović, D. & Blagojević, S. (2021). Rosemary Essential Oils as a Promising Source of Bioactive Compounds: Chemical Composition, Thermal Properties, Biological Activity and Gastronomical Perspectives. Foods, 10(11), 2734.
Miguel, M. G. (2010). Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules, 15(12), 9252–9287.
Mounira, G.M. (2023). Essential Oils and Their Bioactive Molecules: Recent Advantages and New Applications in Essentail Oils – Recent Advances, New Perspectives and Applications ed. Viskelis, J. IntechOpen, doi: 10.5772/intechopen.113406.
Muxika, A., Etxabide, A., Uranga, J., Guerrero, P., & de la Caba, K. (2017). Chitosan as a bioactive polymer: Processing, properties and applications. International Journal of Biological Macromolecules, 105, 1358–1368.
Nazzaro, F., Fratianni, F., De Martino, L., Coppola, R., & De Feo, V. (2013). Effect of essential oils on pathogenic bacteria. Pharmaceuticals, 6(12), 1451–1474.
Negi, A. (2025). Environmental Impact of Textile Materials: Challenges in Fiber–Dye Chemistry and Implication of Microbial Biodegradation. Polymers, 17(7), 871.
Palla F, Bruno M, Mercurio F, Tantillo A, Rotolo V. (2020). Essential Oils as Natural Biocides in Conservation of Cultural Heritage. Molecules, 25(3), 730.
Paolino, B., Sorrentino, M.C. & Pacifico, S. (2024). Greener Solutions for Biodeterioration of Organic-Media Cultural Heritage: Where Are We? Heritage Science, 12, 334.
Pinheiro, A.C., Sequeira, S.O. & Macedo, M.F. (2019). Fungi in Archives, Libraries, And Museums: A Review on Paper Conservation and Human Health. Critical Reviews in Microbiology, 45(5-6), 686-700.
Pop, D.M., Timar, M.C., Varodi, A.M., Beldean, E.C. (2022). An Evaluation of Clove (Eugenia caryophyllata) Essentail Oil as a Potential Alternative Antifungal Wood Protection System for Cultural Heritage Conservation. Maderas, Ciencia y technologia, 24(11), 1-16.
Reale, R., Medeghini, L., & Botticelli, M. (2024). Stealing from Phytotherapy—Heritage Conservation with Essential Oils: A Review, from Remedy to Sustainable Restoration Product. Sustainability, 16(12), 5110.
Riyanto, R., Tri Untari, D., Cahyandaru, N. (2016). Isolation and application of the lemongrass essential oil of Cymbopogon nardus L. as a growth inhibitor of lichens on stone cultural heritage. IOSR Journal of Applied Chemistry 09, 109-117.
Russo, R. & Palla, F. (2023). Plant Essential Oils as Biocides in Sustainable Strategies for the Conservation of Cultural Heritage. Sustainability, 15(11), 8522.
Sala-Luis, A., Oliveira-Urquiri, H., Bosch-Roig, P., & Martín-Rey, S. (2024). Eco-Sustainable Approaches to Prevent and/or Eradicate Fungal Biodeterioration on Easel Painting. Coatings, 14(1), 124.
Sanchis, C.M., Bosch-Roig, P., Moliner, B.C. & Miller, A.Z. (2023). Antifungal Properties of Oregano and Clove Volatile Essential Oils Tested on Biodeteriorated Archaeological Mummified Skin. Journal of Cultural Heritage, 61, 40-47.
Santo, A.P., Agostini, B., Cuzman, O.A., Michelozzi, M., Salvatici, T., Perito, B. (2023). Essential oils to contrast biodeterioration of the external marble of Florence Cathedral. Science of the Total Environment, 877, 162913.
Sasso, S., Scrano, L., Ventrella, E., Bonomo, M.G., Crescenzi, A., Salzano, G. & Bufo, S.A. (2013) Natural Biocides to Prevent the Microbial Growth on Cultural Heritage. Built Heritage 2013 Monitoring Conservation Management, Milan, Italy, 1035-1042.
Shaikh, M.A.J., Gilhotra, R., Pathak, S., Mathur, M., Iqbal, H.M.N., Joshi, N. & Gupta, G. (2021). Current Update on Psyllium and Alginate Incorporate for Interpenetrating Polymer Network (IPN) and Their Biomedical Applications. International Journal of Biological Macromolecules, 191, 432-444.
Sharmeen Jugreet, B., Suroowan S., Kannan Rengasamy, R.R. & Fawzi Mahomoodally, M. (2020). Chemistry, Bioactivities, Mode of Action and Industrial Applications of Essential Oils. Trends in Food Science & Technology, 101, 89-105.
Singh, I.R. & Pulikkal, A.K. (2022). Preparation, Stability and Biological Activity of Essential Oil-Based Nano Emulsions: A Comprehensive Review. OpenNano, 8, 100066.
Soldano, S., Frenda, A., & Borlizzi, P. (2020). Sustainable Re-Use, Preservation and Modern Management of Historical Ruins. RUINS’ Tools & Guidelines. Protection of Cultural Heritage, 10, 115–125.
Szekalska, M., Puciłowska, A., Szymańska, E., Ciosek, P., & Winnicka, K. (2016). Alginate: current use and future perspectives in pharmaceutical and biomedical applications. International Journal of Polymer Science, 2016, 7697031.
Tanasa, F., Nechifor, M. & Teaca, C.A. (2024). Essential Oils as Alternative Green Broad-Spectrum Biocide. Plants, 13(23), 3442.
Tartaro, G., Mateos, H., Schirone, D., Angelico, R., & Palazzo, G. (2020). Microemulsion Microstructure(s): A Tutorial Review. Nanomaterials, 10(9), 1657.
Tomić, A., Šovljanski, O. & Erceg, T. (2023b). Insight on Incorporation of Essential Oils as Antimicrobial Substances in Biopolymer – Based Active Packaging. Antibiotics, 12(9), 1473.
Tomić, A., Šovljanski, O., Nikolić, V., Pezo, L., Aćimović, M., Cvetković, M., Stanojev, J., Kuzmanović, N. & Markov, S. (2023a). Screening of Antifungal Activity of Essential Oils in Controlling Biocontamination of Historical Papers in Archives. Antibiotics, 12(1), 103.
Ural, N. (2021). The Significance of Scanning Electron Microscopy (SEM) Analysis on the Microstructure of Improved Clay: An Overview. Open Geosciences, 13, 197-218.
Vadrucci, M. (2025). Sustainable Cultural Heritage Conservation: A Challenge and an Opportunity for the Future. Sustainability, 17(2), 584.
Vidaković, A., Markov, S., Vučetić, S., Vujović, S., Ducman, V., Hiršenberger, H., Ranogajec, J. (2013). Biosusceptibility of Historical Bricks from the Bač Fortress: Part I. Acta Periodica Technologica 44, 171-180.
Wang Z. & Wang Y. (2016). Tuning amphiphilicity of particles for controllable Pickering emulsion. Materials, 9, 903.