1. Introduction
Antimicrobial resistance (AMR) is a natural evolutionary mechanism by which microorganisms, including bacteria, viruses, fungi, and parasites, develop the capacity to resist the action of antimicrobial drugs. Nevertheless, the widespread misuse and overuse of antibiotics across human medicine, veterinary practices, agriculture, and environmental settings have greatly intensified the pace at which AMR emerges and spreads (World Health Organization, 2023). Antimicrobial resistance is no longer a distant or hypothetical concern; it is already having a significant effect on public health (The Lancet, 2024). In 2021, there were 4.71 million deaths associated with bacterial AMR globally, including 1.14 million deaths directly attributable to bacterial AMR (Naghavi et al. 2024). According to European Centre for Disease Prevention and Control (ECDC) data, the number of deaths attributable to bacterial AMR in the European Union/European Economic Area (EU/EEA) in 2020 was over 35,000 (European Centre for Disease Prevention and Control, 2022). Projections suggest that, if current trends continue, AMR could lead to 1.91 million direct deaths and 8.22 million associated deaths globally by 2050, underscoring the urgent need for coordinated global action (Naghavi et al. 2024). Antibiotic resistance contributes to a significant rise in healthcare costs due to the need for more expensive second- or third-line antimicrobial agents, extended durations of hospitalization, use of advanced medical equipment, and implementation of strict infection control and isolation protocols (Prestinaci et al. 2015).
The growing complexity of AMR challenges conventional epidemiological and clinical tools, exceeding the capacity of standard analytical methods. As a response, in recent years, there has been increasing global momentum to apply advanced information technologies, particularly artificial intelligence (AI) and machine learning (ML), as a scalable and data-driven solution to the evolving threat of antimicrobial resistance (de la Lastra et al. 2024). By leveraging this potential, ML-based solutions are bridging the gap between raw clinical data and timely, evidence-based antimicrobial decision-making, improving both microbiological analysis and empirical antibiotic prescribing, and in general, enabling healthcare systems to move from reactive to proactive, predictive, and personalized responses.
Serbia’s national strategy for the development of artificial intelligence (Republic of Serbia Goverment, 2025) explicitly recognizes the need to apply AI in healthcare, particularly to improve the quality of care and the efficiency of public health interventions. This is the foundation of the ML-ETAR project idea proposed by the team of researchers from the Faculty of Mechanical and Civil Engineering in Kraljevo, University of Kragujevac, the Faculty of Medicine, University of Belgrade, the Faculty of Medical Sciences, University of Kragujevac, and the Faculty of Technical Sciences, University of Priština, which aims to develop a robust and context-sensitive antimicrobial decision-support tool using retrospective data from healthcare institutions in Serbia. By combining local clinical knowledge with advanced computational techniques, the project exemplifies how AI can be responsibly and effectively integrated into national AMR strategies.
2. Current artificial intelligence applications and future prospects in combating AMR
AI, as defined by Stuart Russell and Peter Norvig, leading theorists in artificial intelligence, is “the study of agents that receive percepts from the environment and perform actions” (Russell & Norvig, 2021). In practical terms, AI refers to systems capable of simulating aspects of human intelligence—such as learning, reasoning, and decision-making, in order to solve complex problems. A core subfield of AI – ML focuses on designing algorithms that can learn from historical data and improve their performance over time without being explicitly programmed for each scenario.
ML algorithms are particularly suited to domains where large volumes of complex and heterogeneous data are generated. Microbiology, in particular, produces extensive datasets through routine laboratory testing, microbial culturing, antibiotic susceptibility profiling, molecular diagnostics, and sequencing techniques—all of which contain rich, structured and unstructured information suitable for computational analysis. As noted by LeCun, Bengio, and Hinton—founding figures of modern deep learning, machine learning enables systems to “automatically learn complex representations of data and perform tasks with human-level accuracy or beyond” (LeCun, Bengio & Hinton, 2015). This capability has opened the door to transformative applications in healthcare, including microbiology, where such systems can detect subtle patterns across large, heterogeneous datasets and support high-stakes clinical decisions.
In healthcare, ML has already been applied to diagnostic classification, risk prediction, treatment selection, and outbreak surveillance. Importantly, the role of AI is not to replace clinical judgment, but to augment it—serving as a decision-support tool that enhances accuracy, speed, and personalization of care. Compared to other domains of healthcare—such as medical imaging, oncology, and risk prediction—where AI and ML have been adopted since the early 2010s, the application of these technologies in antimicrobial resistance (AMR) has emerged more recently (Liu et al. 2024). This delayed integration is largely due to challenges related to fragmented data sources, limited availability of large standardized datasets, and the multifactorial nature of resistance development. However, since around 2020, there has been a notable increase in AI-driven AMR research and deployment, supported by improved data infrastructure and growing interdisciplinary collaboration.
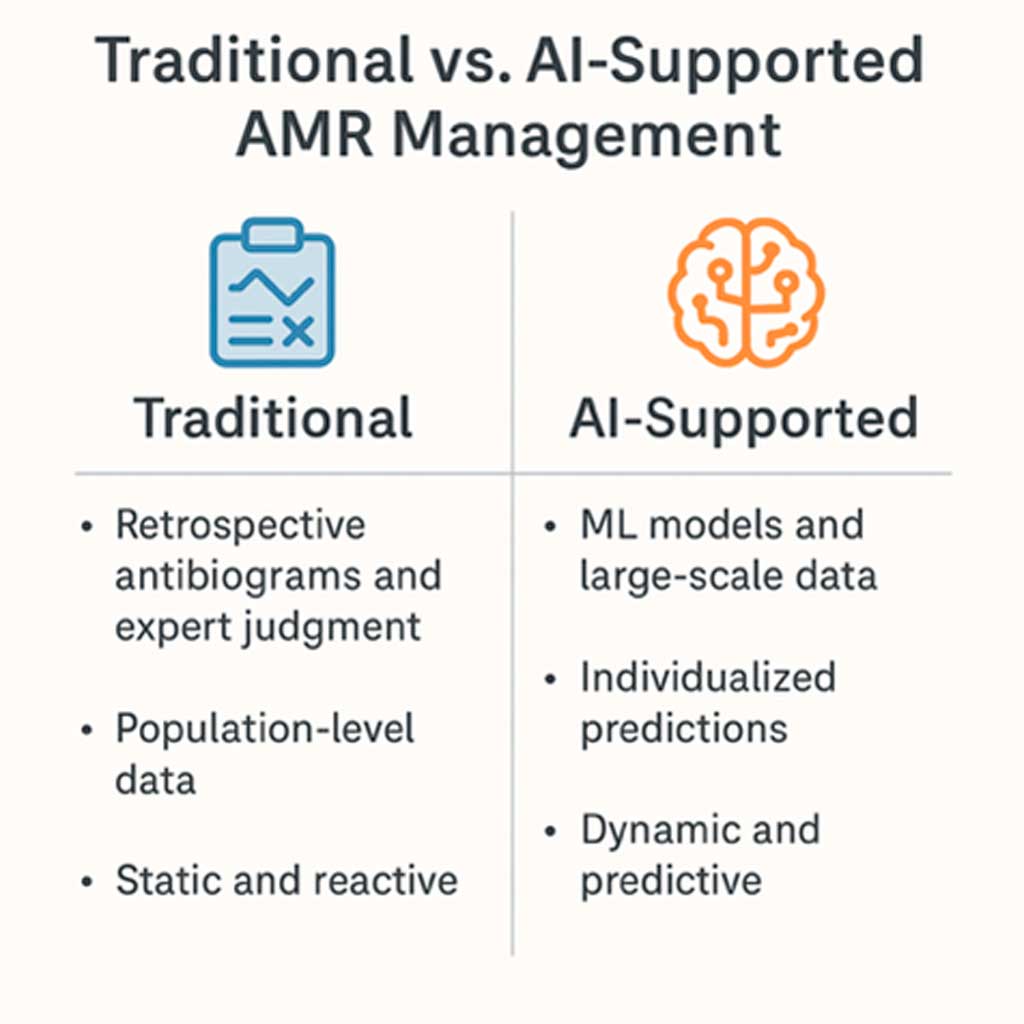
Figure 1. Traditional vs. AI-supported AMR management
The benefits and limitations of this approach were critically reviewed by Pérez de la Lastra et al. (2024). Among the key advantages, the authors highlight: early detection of resistance trends, optimization of empirical treatment, integration of large and diverse datasets (e.g., clinical, genomic, environmental), and potential to inform public health policy in real time. However, they also emphasize notable challenges and limitations: data fragmentation and poor interoperability between systems, lack of standardization and validation of AI models across institutions, concerns regarding explainability of complex ML algorithms (often described as “black-box” models), and ethical and legal considerations related to patient data privacy and algorithmic bias. As they conclude, while the promise of AI in AMR is clear, its successful implementation depends not only on multidisciplinary collaboration but on robust data governance and regulatory frameworks that ensure safe, transparent, and equitable deployment of these technologies in real-world clinical settings.
These observations underscore the need to reflect on how AI-supported approaches differ from traditional AMR management paradigms—not just technologically, but conceptually and operationally, Figure 1. Traditionally, AMR surveillance has relied on retrospective, population-level data aggregated into annual antibiograms. These summaries provide valuable insights into local resistance patterns but lack temporal granularity, personalization, and predictive capacity. Clinical decision-making in this paradigm is often reactive, based on past trends and expert interpretation rather than individualized risk profiles or forward-looking models. Furthermore, traditional approaches struggle to keep pace with the dynamic and multifactorial nature of AMR, particularly in high-acuity hospital settings where timeliness and precision are critical.
In contrast, AI-enhanced systems, particularly those leveraging ML, represent a paradigm shift. These models can ingest vast, heterogeneous datasets—including electronic health records, antimicrobial usage logs, resistance patterns, and microbiological data—and use them to generate predictive, individualized insights. For example, recent studies have shown that ML models such as extreme gradient boosting (XGBoost) outperform traditional statistical baselines in forecasting hospital-level resistance prevalence (Corbin et al. 2022; Vihta et al. 2024). These models can identify subtle, non-linear relationships between variables and update predictions as new data becomes available, enabling a more adaptive and data-driven approach to antimicrobial stewardship.
From this perspective, AI does not simply enhance traditional tools—it transforms them, allowing healthcare systems to transition from descriptive to prescriptive analytics. Instead of asking “What was the resistance rate last year?”, clinicians and microbiologists can now ask “What is the likely resistance risk for this patient today—and how might that change next month?”
This evolution aligns with the broader shift toward precision medicine and proactive infection control, where timely and context-specific decisions are paramount.
2.1. Application of artificial intelligence in microbiological diagnostics
According to numerous recent studies, AI/ML has demonstrated considerable potential in advancing microbiological diagnostics, supporting the discovery of novel antimicrobial agents, and refining the prediction of empirical antibiotic therapies.
2.1.1 Application of Machine Learning for AMR Prediction
Conventional methods, though effective, are slow and labour-intensive, often delaying targeted treatment for 48–72 hours. This can lead to extended use of broad-spectrum antibiotics. Therefore, fast, accurate, and cost-effective diagnostics are essential to improve antimicrobial use and reduce selective pressure (Benkova, Soukup and Marek, 2020).
The integration of ML techniques with MALDI-TOF mass spectrometry data has emerged as a promising strategy for the rapid and accurate identification of AMR. This approach enables direct detection of resistant phenotypes from clinical isolates, thereby significantly reducing diagnostic turnaround times. Algorithms such as random forest, support vector machines, and gradient boosting have shown robust performance in predicting resistance in clinically relevant pathogens, including Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus. (Weis et al. 2022; Xu et al. 2025; López-Cortés et al. 2024).
Whole-genome sequencing-based antimicrobial susceptibility testing (WGS-AST) facilitates rapid and reliable prediction of known resistance phenotypes while supporting robust surveillance through comprehensive genomic data. By analyzing WGS-AST datasets, machine learning algorithms can identify genetic determinants of resistance. For individual antibiotics, optimized ML models have demonstrated a 2% to 24% increase in sensitivity compared to traditional rules-based approaches (Su, Satola, and Read, 2019).
A study by Moradigaravand et al. (2018) showed that resistance in E. coli can be accurately predicted from whole-genome data without prior knowledge of specific mechanisms, underscoring the importance of integrating genomic and epidemiological information within WGS-AST frameworks.
Similarly, a study conducted in China applied supervised learning to predict β-lactam resistance in Streptococcus pneumoniae using partial pbp2x and pbp2b gene sequences. Models trained on MIC-labeled and unlabeled data accurately predicted resistance to cefuroxime and amoxicillin, with validation performed on engineered mutants and clinical isolates, demonstrating the practical value of ML-based sequence analysis in AMR diagnostics (Zhang et al. 2020). Expanding on these approaches, recent work on Mycobacterium tuberculosis employed deep convolutional neural networks (CNNs) to predict resistance to 13 antibiotics using 18 genomic loci, achieving area under the curve (AUCs) between 80.1% and 99.5% and outperforming previous methods in sensitivity and specificity. Saliency analyses further revealed novel resistance-associated sites, improving model interpretability and reinforcing the clinical utility of deep learning in both resistance prediction and functional variant discovery (Green, et al. 2022).
2.1.2 Application of Artificial Intelligence in Antimicrobial Drug Development
AI has been integrated into various stages of drug development (Gupta et al. 2021). For example, Roberts et al. (2022) employed AI-driven approaches to develop a synthetic lipopeptide effective against multidrug-resistant Gram-negative pathogens, demonstrating the potential of AI to accelerate the development of therapeutics targeting critical-priority bacteria. In addition to facilitating the identification of novel chemical scaffolds, machine learning approaches are expected to play a crucial role in predicting antimicrobial resistance patterns and drug metabolism (Panjla et al. 2024).
2.1.3 Application of Machine Learning in Antimicrobial Stewardship and Surveillance
Artificial intelligence systems contribute to improved antibiotic stewardship in hospital settings by tracking prescribing behaviours and identifying instances of overuse or inappropriate use. By analysing prescription patterns and detecting anomalies, these systems enable targeted educational efforts or interventions aimed at optimizing antimicrobial prescribing practices (Pinto-de-Sá et al. 2024). Kanjilal et al. (2020) developed a machine learning model trained on electronic health record data from a local hospital to estimate the likelihood of resistance to first- and second-line antibiotics in cases of uncomplicated urinary tract infections (UTIs). Based on these predictions, the system recommended the narrowest-spectrum antibiotic to which the isolate was likely susceptible. Compared to clinician prescribing patterns, the algorithm reduced the use of second-line antibiotics by 67% and inappropriate antibiotic therapy—defined as prescribing an agent to which the pathogen is resistant—by 18%, underscoring its clinical potential to improve empiric treatment decisions.
A multi-site study from Stanford and Boston hospitals proposes a novel ML framework that generates personalized antibiograms by leveraging electronic health records and microbiological data. Unlike traditional antibiograms that offer population-level resistance data, this approach provides individualized antibiotic susceptibility profiles tailored to the patient’s clinical and microbiological context. The models were trained and evaluated on 8,122 infections from Stanford Hospital and 15,803 urinary tract infections from hospitals in Boston. In the Stanford cohort, ML models achieved 85.9% coverage of infections (fraction of infections covered by treatment), comparable to clinicians (84.3%, p = 0.11), while in the Boston cohort, coverage reached 90.4%, significantly outperforming clinicians (88.1%, p < 0.0001). The analysis focused on four broad-spectrum antibiotics—vancomycin, piperacillin-tazobactam, ciprofloxacin, and nitrofurantoin—and demonstrated multiple opportunities for de-escalation without compromising coverage. For example, 69% of vancomycin + piperacillin-tazobactam therapies could be narrowed to piperacillin-tazobactam alone, and 93% of ciprofloxacin prescriptions could be safely replaced with nitrofurantoin. These results highlight the potential of AI to support precision medicine in antimicrobial prescribing and enhance treatment outcomes (Corbin et al. 2022).
A notable example of an AI application in AMR management is also the study conducted in the United Kingdom by researchers who developed ML models to predict future antimicrobial resistance prevalence at the hospital level. Using comprehensive national data from 138 hospital groups (known as NHS Trusts) collected over a six-year period (2016–2022), the study focused on 22 clinically important pathogen–antibiotic combinations, including resistant strains of E. coli, K. pneumoniae, and Pseudomonas aeruginosa. The models incorporated a wide range of predictors—such as historical AMR prevalence trends, hospital-level antibiotic consumption, and contextual variables like patient load and institutional size—demonstrating that these factors collectively enhance the predictive performance of the system (Vihta et al. 2024). Among the six models tested, XGBoost consistently outperformed traditional forecasting methods such as previous value taken forwards (PVTF), difference taken forwards, and linear trend forecasting (LTF), with an average reduction in mean absolute error (MAE) of 1–3 percentage points. In specific cases, such as P. aeruginosa and ceftazidime, the MAE was reduced from 6% to 4%, representing a 33% relative improvement. These performance gains were particularly pronounced in hospital settings with large year-to-year variations in resistance prevalence, where XGBoost showed superiority in nearly all pathogen–antibiotic combinations. XGBoost is a state-of-the-art machine learning algorithm that builds a series of simple decision trees, where each new tree corrects the errors of the previous ones. It is especially effective in analysing complex, high-dimensional datasets, such as those encountered in microbiological surveillance systems.
Yang et al. (2023) go beyond proof-of-concept to deliver an interpretable ML‑based clinical decision support system for predicting antibiotic resistance in complex urinary tract infections. Trained on a large cohort of complicated UTI cases and externally validated on an independent uncomplicated UTI cohort, the tool demonstrated high predictive accuracy—exceeding 85–90% for four commonly used antibiotics: nitrofurantoin, co‑trimoxazole, ciprofloxacin, and levofloxacin. The authors provided detailed performance metrics, including area under the receiver operating characteristic curve (AUROC) and area under the precision-recall curve (AUPRC) with 95% confidence intervals, confirming the clinical robustness of the predictions. Importantly, the models were designed with interpretability in mind, using Shapley additive explanations SHAP values to highlight the most influential clinical variables for each prediction.
This and similar scientific results underline the potential of ML-based approaches to support data-driven AMR surveillance and guide antimicrobial stewardship efforts, highlighting the growing maturity of AI in clinical microbiology and its relevance for strengthening antibiotic stewardship programs.
3. Pioneering machine learning in empirical antimicrobial therapy in Serbia
Given that ML algorithms are highly data-dependent, a cornerstone of ML approach to AMR management is the establishment of a high-quality, structured dataset derived from existing health information infrastructure. Fortunately, many healthcare institutions in Serbia already utilize electronic health records (EHRs), which offer a valuable foundation for advanced data collection and integration. When properly aggregated and standardized, EHR data that identifies relevant clinical parameters from microbiological laboratories, biochemical analyses, and patient histories can be used to train ML models. Once trained and optimized, models will be able to identify resistance patterns and predict the most effective empirical antibiotic therapies. The process of building such models requires close collaboration between data scientists and microbiology experts. Domain knowledge is crucial not only for labelling and validating input variables but also for interpreting the model’s outputs and refining its predictions.
In this context, an initiative has been launched by a team of researchers from multiple faculties — including the Faculty of Mechanical and Civil Engineering in Kraljevo, University of Kragujevac, the Faculty of Medicine, University of Belgrade, the Faculty of Medical Sciences, University of Kragujevac, and the Faculty of Technical Sciences, University of Priština — with the aim of developing a comprehensive, scalable decision-support tool for empirical antimicrobial therapy, built on domestic data and tailored to the real needs of Serbia’s healthcare system. The project, Machine Learning Utilization for Data-Driven Empirical Therapy and Antimicrobial Resistance Management, ML-ETAR, integrates expertise in microbiology, pharmacology, data science, and software engineering, and relies on retrospective data from six healthcare institutions across Serbia to train machine learning models that will inform more precise and context-aware antibiotic prescribing.
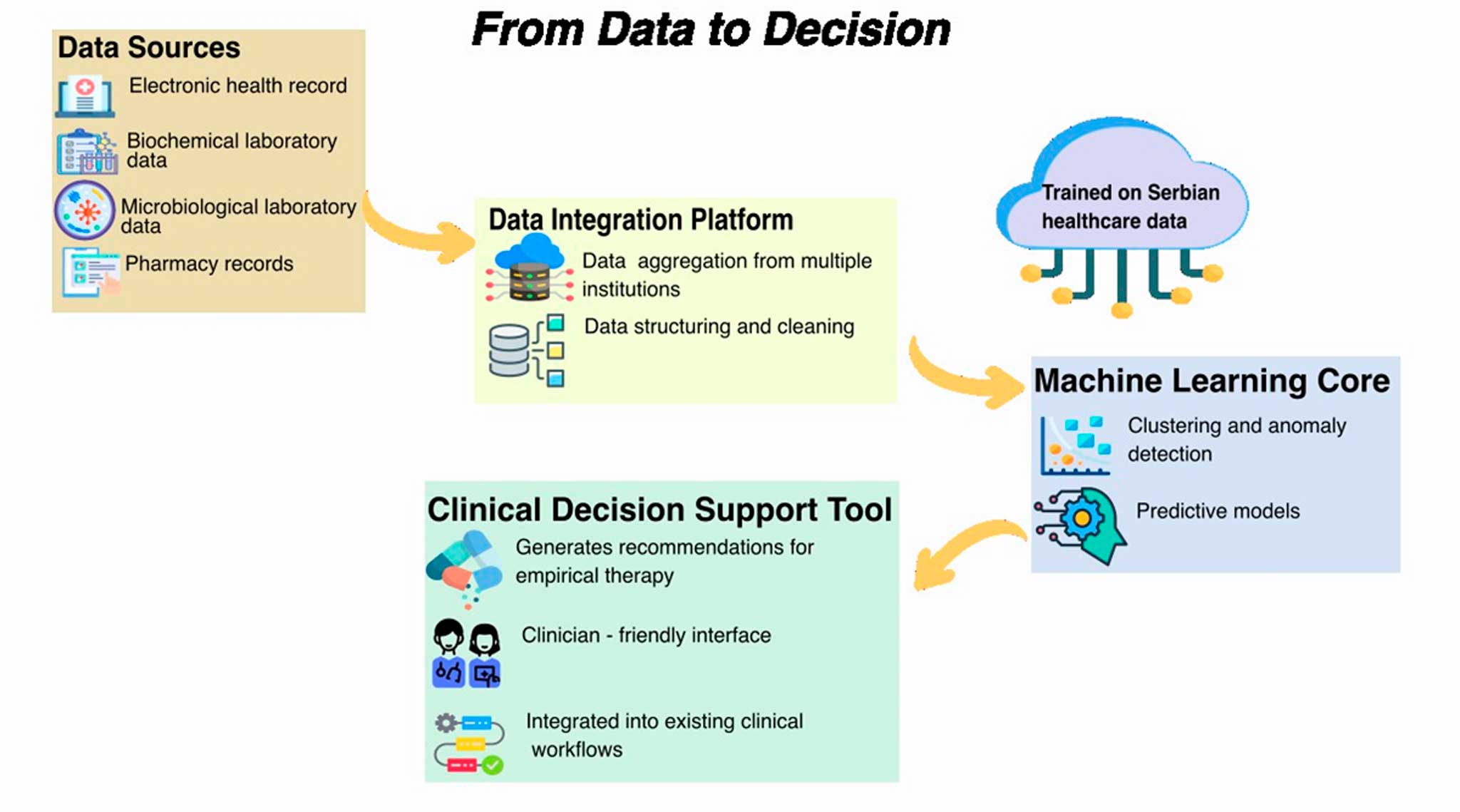
Figure 2. ML supported AMR management – project ML-ETAR
Figure 2. illustrates the logical flow of project – from data to clinical decision – and outlines the core components of the system we are developing to combat AMR through AI. The process begins with four key data sources: electronic health records, biochemical laboratory data, microbiological laboratory data, and pharmacy/prescription records. During the initial phase of the project, data were collected from six healthcare institutions across Serbia, selected to represent a broad range of geographic and demographic diversity, a critical factor for building a representative dataset. These datasets are funnelled into a Data Integration Platform, which enables aggregation across institutions, standardization of formats, and data cleaning. This step was particularly challenging due to the heterogeneity of information systems used in Serbian healthcare facilities. The integrated data feeds into the Machine Learning Core, where clustering and anomaly detection techniques are applied alongside the development of predictive models capable of recommending optimal empirical antibiotic therapy. Crucially, the models are trained on Serbian health data, making them context-sensitive and tailored to the realities of the local healthcare system.
The final output is a Clinical Decision Support Tool that provides empirically grounded recommendations. Designed with a clinician-friendly interface, it integrates smoothly into existing clinical workflows without requiring significant changes from the medical staff.
The overarching goal of this initiative is to enable more accurate and personalized empirical treatment, reduce the overuse of broad-spectrum antibiotics, and contribute to long-term AMR control through a scalable and intelligent digital solution.
4. Conclusion
The integration of AI in combating AMR offers a transformative opportunity to modernize surveillance, improve patient outcomes, and optimize antibiotic use. The interdisciplinary initiative launched by researchers from several Serbian faculties presents a forward-thinking model for how data, domain expertise, and intelligent algorithms can be brought together to tackle AMR challenges. By building a scalable, context-sensitive decision-support system grounded in local healthcare realities, this project stands out as a pioneering effort with strong potential for long-term scientific, societal, and healthcare impact. Its methodological approach could serve as a blueprint for similar initiatives in other countries in the region facing high AMR burdens.
Acknowledgment: The authors acknowledge support of the Ministry of Science, Technological Development and Innovation of the Republic of Serbia, contract number [451-03-137/2025-03/200108 (Faculty of Mechanical and Civil Engineering in Kraljevo, University of Kragujevac)]. This research is in line with the strategic plan of the Republic of Serbia 2030, particularly in the section 2.2.2 Development of human resources, goal 3: Good health
References
Benkova, M., Soukup, O. and Marek, J. (2020) ‘Antimicrobial susceptibility testing: currently used methods and devices and the near future in clinical practice’, Journal of Applied Microbiology, 129(4), pp. 806–822. Available at: https://doi.org/10.1111/jam.14704.
Cherkaoui, A. et al. (2020) ‘Impact of Total Laboratory Automation on Turnaround Times for Urine Cultures and Screening Specimens for MRSA, ESBL, and VRE Carriage: Retrospective Comparison With Manual Workflow’, Frontiers in Cellular and Infection Microbiology, 10. Available at: https://doi.org/10.3389/fcimb.2020.552122.
Corbin, C.K. et al. (2022) ‘Personalized antibiograms for machine learning driven antibiotic selection’, Communications Medicine, 2(1), p. 38. Available at: https://doi.org/10.1038/s43856-022-00094-8.
de la Lastra, J.M.P., Wardell, S.J.T., Pal, T., de la FuenteNunez, C. & Pletzer, D., 2024. From Data to Decisions: Leveraging Artificial Intelligence and Machine Learning in Combating Antimicrobial Resistance – a Comprehensive Review. Journal of Medical Systems, 48(1), p.71. doi:10.1007/s10916-024-02089-5
European Centre for Disease Prevention and Control. (2022). Assessing the health burden of infections with antibiotic-resistant bacteria in the EU/EEA, 2016-2020. Stockholm: ECDC.
Green, A.G., Yoon, C.H., Chen, M.L. et al. (2022). A convolutional neural network highlights mutations relevant to antimicrobial resistance in Mycobacterium tuberculosis. Nat Commun 13, 3817 https://doi.org/10.1038/s41467-022-31236-0
Gupta, R., Srivastava, D., Sahu, M., Tiwari, S., Ambasta, R.K. and Kumar, P. (2021). Artificial intelligence to deep learning: machine intelligence approach for drug discovery. Molecular Diversity, [online] 25(3), pp.1–46. doi:https://doi.org/10.1007/s11030-021-10217-3.
Jameela, T. et al. (2022) ‘Deep Learning and Transfer Learning for Malaria Detection’, Computational Intelligence and Neuroscience. Edited by S. Roy, 2022, pp. 1–14. Available at: https://doi.org/10.1155/2022/2221728.
Kanjilal, S., Oberst, M., Boominathan, S., Zhou, H., Hooper, D. C., & Sontag, D. (2020). A decision algorithm to promote outpatient antimicrobial stewardship for uncomplicated urinary tract infection. Science Translational Medicine, 12(568), eaay5067. https://doi.org/10.1126/scitranslmed.aay5067
The Lancet (2024). Antimicrobial resistance: an Agenda for All. Lancet, 403(10442). doi:https://doi.org/10.1016/s0140-6736(24)01076-6.
LeCun, Y., Bengio, Y., & Hinton, G. (2015). Deep learning. Nature, 521(7553), 436–444. https://doi.org/10.1038/nature14539
Liu, GY., Yu, D., Fan, MM. et al. Antimicrobial resistance crisis: could artificial intelligence be the solution?. Military Med Res 11, 7 (2024). https://doi.org/10.1186/s40779-024-00510-1
López-Cortés, X.A., Manríquez-Troncoso, J.M., Kandalaft-Letelier, J. and Cuadros-Orellana, S. (2024). Machine learning and matrix-assisted laser desorption/ionization time-of-flight mass spectra for antimicrobial resistance prediction: A systematic review of recent advancements and future development. Journal of Chromatography A, 1734, p.465262. doi:https://doi.org/10.1016/j.chroma.2024.465262.
Marletta, S. et al. (2023) ‘Artificial intelligence-based tools applied to pathological diagnosis of microbiological diseases’, Pathology – Research and Practice, 243, p. 154362. Available at: https://doi.org/10.1016/j.prp.2023.154362.
Mathison, B.A. et al. (2020) ‘Detection of Intestinal Protozoa in Trichrome-Stained Stool Specimens by Use of a Deep Convolutional Neural Network’, Journal of Clinical Microbiology. Edited by B.S. Pritt, 58(6). Available at: https://doi.org/10.1128/jcm.02053-19.
Moradigaravand, D. et al. (2018) ‘Prediction of antibiotic resistance in Escherichia coli from large-scale pan-genome data’, PLOS Computational Biology. Edited by A.E. Darling, 14(12), p. e1006258. Available at: https://doi.org/10.1371/journal.pcbi.1006258.
Naghavi, M., Vollset, S.E., Ikuta, K.S., Swetschinski, L.R., Gray, A.P., Wool, E.E., Robles Aguilar, G., Mestrovic, T., Smith, G., Han, C., Hsu, R.L., Chalek, J., Araki, D.T., Chung, E., Raggi, C., Gershberg Hayoon, A., Davis Weaver, N., Lindstedt, P.A., Smith, A.E. and Altay, U. (2024). Global Burden of Bacterial Antimicrobial Resistance 1990–2021: a Systematic Analysis with Forecasts to 2050. The Lancet, [online] 404(10459), pp.1199–1226. doi:https://doi.org/10.1016/s0140-6736(24)01867-1.
Panjla, A. et al. (2024) ‘Applying Machine Learning for Antibiotic Development and Prediction of Microbial Resistance’, Chemistry – An Asian Journal [Preprint]. Available at: https://doi.org/10.1002/asia.202400102.
Prestinaci, F., Pezzotti, P., & Pantosti, A. (2015). Antimicrobial resistance: a Global Multifaceted Phenomenon. Pathogens and Global Health, 109(7), 309–318. https://doi.org/10.1179/2047773215y.0000000030
Russell SJ, Norvig P. Artificial Intelligence: A Modern Approach. 4th ed. Pearson; 2021.
Republic of Serbia Goverment (no date) Artificial Intelligence Development Strategy in the Republic of Serbia for the period from 2025 to 2030. Available at: https://nitra.gov.rs/images/vesti/2025/2025-01-10-link/Strategija%20razvoja%20vestacke%20inteligencije%202025-2030.pdf.
Roberts KD, Zhu Y, Azad MAK, Han ML, Wang J, Wang L, et al. A synthetic lipopeptide targeting top-priority multidrug-resistant Gram-negative pathogens. Nat Commun. 2022;13(1):1625
Smith, K.P. and Kirby, J.E. (2020) ‘Image analysis and artificial intelligence in infectious disease diagnostics’, Clinical Microbiology and Infection, 26(10), pp. 1318–1323. Available at: https://doi.org/10.1016/j.cmi.2020.03.012.
Song, Y. et al. (2017) ‘Segmentation, Splitting, and Classification of Overlapping Bacteria in Microscope Images for Automatic Bacterial Vaginosis Diagnosis’, IEEE Journal of Biomedical and Health Informatics, 21(4), pp. 1095–1104. Available at: https://doi.org/10.1109/jbhi.2016.2594239.
Su, M., Satola, S.W. and Read, T.D. (2019) ‘Genome-Based Prediction of Bacterial Antibiotic Resistance’, Journal of Clinical Microbiology. Edited by A.J. McAdam, 57(3). Available at: https://doi.org/10.1128/jcm.01405-18.
Vihta, K.-D. et al. (2024) ‘Predicting future hospital antimicrobial resistance prevalence using machine learning’, Communications Medicine, 4(1), p. 197. Available at: https://doi.org/10.1038/s43856-024-00606-8.
Weis, C., Cuénod, A., Rieck, B., Dubuis, O., Graf, S., Lang, C., Oberle, M., Brackmann, M., Søgaard, K.K., Osthoff, M., Borgwardt, K. and Egli, A. (2022). Direct antimicrobial resistance prediction from clinical MALDI-TOF mass spectra using machine learning. Nature Medicine. doi:https://doi.org/10.1038/s41591-021-01619-9.
World Health Organization (2023). Antimicrobial Resistance. [on line] World Health Organization. Available at: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
Xu, X., Wang, Z., Lu, E., Lin, T., Du, H., Li, Z. and Ma, J. (2025). Rapid detection of carbapenem-resistant Escherichia coli and carbapenem-resistant Klebsiella pneumoniae in positive blood cultures via MALDI-TOF MS and tree-based machine learning models. BMC Microbiology, 25(1). doi:https://doi.org/10.1186/s12866-025-03755-5.
Yang, J., Eyre, D. W., Lu, L., & Clifton, D. A. (2023). Interpretable machine learning-based decision support for prediction of antibiotic resistance for complicated urinary tract infections. Antimicrobials and Resistance, 1(1), Article 14. https://doi.org/10.1038/s44259-023-00015-2
Zhang, C., Ju, Y., Tang, N., Li, Y., Zhang, G., Song, Y., Fang, H., Yang, L., & Feng, J. (2020). Systematic analysis of supervised machine learning as an effective approach to predicate β-lactam resistance phenotype in Streptococcus pneumoniae. Briefings in Bioinformatics, 21(4), 1347–1355. https://doi.org/10.1093/bib/bbz056