1. Introduction
Due to their pronounced biodeteriorative potential, fungi, both non-lichenized and lichenized forms, are considered the main causes of decay of all types of historic works of art, from the oldest and most pre- cious rock art caves, such as the caves of Lascaux in France, to art made of modern materials exhibited in public museums or stored in depots and private art collections (Sterflinger & Piñar, 2013). Since fungi are ubiquitous organisms that possess enormous en- zymatic activity and ability to grow at low aw values, combined with the fact that many of them are toxi- genic, allergenic and pathogenic, their proliferation on cultural heritage not only results in unacceptable structural and aesthetic impairments, but likewise has consequences for the occupational safety of con- servators, restorers and other personnel responsible for their upkeep (Sterflinger, 2010). Discovering ade- quate control method for suppression of fungal infes- tation of works of art, with minimal environmental impact, is a considerable problem for the profession- als responsible for the conservation and preservation of cultural heritage (Fonseca et al., 2010). The pre- vention of mould growth and decontamination of infected artefacts is high expenditure for institutions responsible for their preservation with estimates that at the annual level world loss of non-food materials due to fungal attack is approximately 40 billion USD (Allsopp, 2011). To ensure sustainable and lasting re- moval of fungal growth and conservation of biodete- riorated cultural properties only methods and prod- ucts deemed acceptable by available scientific knowl- edge may be applied: mechanical, physical, and (bio) chemical (Kakakhel et al., 2021). Mechanical clean- ing, manually or via tools such as scalpels, spatulas, and vacuum cleaners, and physical methods, using laser, heat, UV radiation, and low frequency electri- cal systems, are less commonly used for conservation of heritage materials compared to biochemical meth- ods i.e. application of biocides to eradicate fungal growth (Scheerer et al., 2009; Allsopp, 2011). Bio- cides are one of the most effective methods to control fungal growth in the process of cultural heritage con- servation (Kakakhel et al., 2021). Nowadays, there is an assortment of traditional and low-cost chemical inorganic and organic fungicides available on the market that are highly effective in suppression of a broad range of fungi because of their acute toxicity. Many are synthetic mixtures formed by quaternary ammonium compounds, other nitrogen-containing compounds, urea, benzalkonium chloride, phenol derivatives or other molecules (Caldeira et al., 2021). In the Lascaux Cave in France, outbreak of Fusari- um solani was controlled over a three year period with varying degrees of success using biocides that consisted mainly of chemicals such as quaternary ammonium, benzalkonium chloride, 2-octyl-2H- isothiazol-3-one, and Parmetol (Martin-Sanchez et al., 2013). However, due to their toxicity for oper- ators and the environment, non-selective mode of action, abrasiveness, low long-term effectiveness and promotion of resistance, use of chemical biocides in heritage repositories and outdoor environment is increasingly discouraged (Cappitelli et al., 2020). In some instances, such as with use of ethylene oxide for fumigation of fungal-infested heritage objects, out- right ban was instigated in numerous countries be- cause of carcinogenic and mutagenic features of this gas (Nugari and Salvadori, 2003). This is of great im- portance, since conservators are known to be among those most frequently exposed to toxic biocides (Var- nai et al., 2011).

Figure 1. Ad-hoc-constructed aromatic chamber for in situ application of Boswellia carteri essential oil vapors aimed at cleansing fungal-contaminated air within the old Church of the Holy Ascension (Serbia).
The need for eco-friendly alternatives to toxic biocides has directed the research in last decade towards potential application of natural products, mainly derived from plants – essential oils, crude extracts, and pure compounds, in the process of conservation of works of art. For example, fumes created by burning Cinnamomum verum bark and Tamarix nilotica leaves completely sterilized archival repository of National Records and Archives (Tanta, Egypt) and left no residues or color/structure alterations of treated documents (Tayel et al., 2016), while Boswellia carteri essential oil vapors and burn incense fume reduced air-borne viable fungal counts by up to 80% when in situ applied in the old Church of the Holy Ascension (Serbia) resulting in no alterations on the mural painting surfaces in the process (Fig. 1) (Ljaljević Grbić et al., 2018). Furthermore, volatile compounds of Origanum vulgare and Thymus vulgaris were applied in ad- hoc-assembled „clean chambers“ to treat Aspergillus flavus-infested wooden artwork (Palla et al., 2020). Despite the many advantages, such as efficiency against a broad range of fungi, ease of application and generally environmentally friendly nature, up to date, only few products are available on the market. The main drawback of using natural compounds of plant origin is that their chemical composition and subsequent antifungal activity are largely dependent on phenological phase and geographical location, as well as the extraction method. As chemical characterization is needed every time and usually low quantities of phytochemicals are obtained during extractions their application in situ can be quite costly (Cappitelli et al., 2020). In addition, there is a lack of data regarding the efficiency over time, interference of phytochemicals with heritage materials, as well as other potential hazards (Fidanza and Caneva, 2019).
2. Biocontrol potential of beneficial bacteria
Because of environmental and healthcare problems related to the use of chemical biocides, as well as due to limitations that result in use of natural compounds of plant origin, new paradigm in bioconservation studies relates to research on biocontrol potential of bacterial isolates (Fig. 2), not least due to possibility of implementing biotechnological approaches to obtain sufficient amounts of bioactive compounds. In one of the pioneer studies in this field, full culture of Burkholderia gladioli pv. agaricicola ICMP 11096 demonstrated in vitro to possess higher antifungal activity against fungi from genera Aspergillus, Coprinellus, Fusarium, Penicillium, and Stemphylium, isolated from San Vito Bridge in Potenza and Della Vecchia Bridge in Campomaggiore, compared to cell-free broth (Sasso et al., 2013). Furthermore, Bacillus– based bioformulation, containing spores of B. subtilis, B. pumilus, and B. megaterium, in an in vitro experiment demonstrated almost complete growth inhibition of Alternaria, Aspergillus, Cladosporium and Penicillium fungi, isolated from the 17th century easel painting “Incoronazione della Vergine” by Carlo Bononi (Caselli et al., 2018). Observed inhibitory effect was most likely achieved via non-specific competitive antagonism, since growth inhibition was also demonstrated against isolated bacteria from Bacillus and Staphylococcus genera.
Very extensive work with Bacillus strains, as source of bioactive compounds applicable in conservation, was done in recent years by Silva and her coworkers from HERCULES Laboratory of Évora University in Portugal. In two of their studies, antifungal potential of three Bacillus strains (CCMI 1051, CCMI 1052, and CCMI 1053) was evaluated against fungi of genera Alternaria, Cladosporium, Fusarium, Mucor, and Penicillium, commonly found in biodeteriorated heritage materials. Major growth inhibition was demonstrated for strain CCMI1053 against Penicillium sp. and Cladosporium sp., while characterizationofitsbioactivemetabolites,vianuclear magnetic resonance and liquid chromatography- electrospray ionization-tandem mass spectrometry, indicated lipopeptides iturin A, surfactin and fengycin presumably responsible for documented activity (Silva et al., 2015; Silva et al., 2016b). High growth inhibition demonstrated for strain Bacillus sp. CCMI1053 was additionaly confirmed against Aspergillus, Fusarium, and Penicillium fungi isolated from biodeteriorated mural paintings (Silva et al., 2018). Likewise, in this research, production of bioactive lipopeptides was shown to be increased in Bacillus sp. CCLBH 1053 culture supplemented with peptone and heat activated, indicating that knowledge on the physiological response of Bacillus to nutrient supplementation can help improve the production of antifungal compounds in biotechnological processes. Furthermore, secondary metabolites, dubbed as BEVOTECH 3, BEVOTECH 4, and BEVOTECH 5, produced by Bacillus sp. CCLBH 1053, demonstrated very high antifungal activity against biodeteriogenic fungi of genera Alternaria, Fusarium, Mucor, and Penicillium, isolated from deteriorated mural paintings (Silva et al., 2019). Since good antifungal property was also demonstrated for leaf ethanolic extract of Pouteria ramiflora, against biodeteriogenic fungi of genera Aspergillus, Cladosporium, Fusarium, Penicillium and Ulocladium, authors proposed a combination of BEVOTECH metabolites and P. ramiflora ethanolic extract should be considered when formulating novel effective green biocide of increased activity compared to individual components. Great antifungal potential of Bacillus spp.-produced biosurfactant lipopeptides dubbed BEVOTECH 11, 14 and 16 was demonstrated againt fungi isolated from mural paintings from Casas Pintadas in Évora and from Convent of Christ (Acremonium, Aspergillus, Cladosporium, Cryptococcus, Penicillium species and undetermined filamentous fungi and yeast), with BEVOTECH 11 sometimes having higher activity compared to commercial biocides Preventol PN® 0.1%, Panacide and Linquad (Rosado et al., 2017, 2019). Bacillus amyloliquefaciens CCM I1051 demonstrated greater in vitro activity compared to commercial biocides Panacide and NEW DES against Aspergillus, Cladosporium, Mucor, and Penicillium fungi isolated from four 19th century easel paintings of Giorgio Marini (Salvador et al., 2016). In another study, out of 21 tested Bacillus strains, four strains with high level of morphological and biochemical similarity with B. amyloliquefaciens, B. pumilus, and B. subtilis showed the ability to suppress biodeteriogenic filamentous fungi and yeasts isolated from heritage environments, naimly species of Alternaria, Aspergillus, Cladosporium, Fusarium, Mucor, Penicillium, and Rhodotorula genera. For all four strains presence of iturinic genes (ituA, ituB, ituC, and ituD) of various degrees of expression, responsible for the iturinic compounds biosynthesis, was demonstrated. Thus, according to authors these iturin-producing strains have greater potential to be utilized as natural green bioformulations in biodeterioration mitigation strategies (Silva et al., 2017).
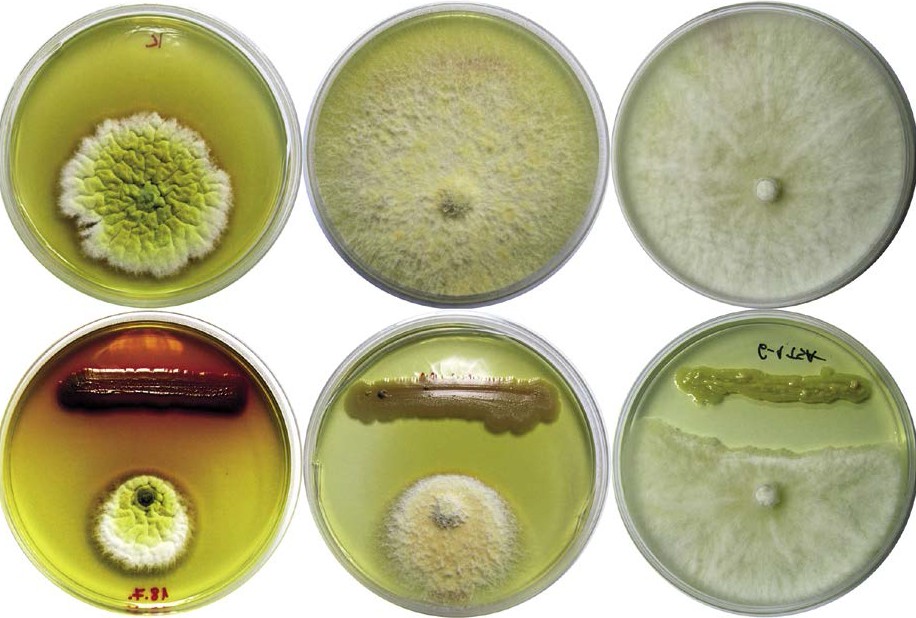
Figure 2. Biocontrol potential of beneficial bacteria determined in dual cultivation assay.
In their latest research, Dimkić et al. (2023) studied bacterial communities within the cave Church of Sts. Peter and Paul in Serbia in order to characterize and select the best antagonistic strains to be potentially used for suppresion of fungal infestation of deteriorated fresco painting and wooden iconostasis in the investigated church. Out of 36 evaluated autochthonous isolates four, determined as Bacillus altitudinis 6-1 TSA, Chryseobacterium viscerum 7-15 (614), Streptomyces anulatus 1-3 MM, and Streptomyces sp. 11-11 MM, represent excellent candidates for developing biocontrol strategies since they were able to inhibit mycelial growth of 10 autochthonous biodeteriogenic fungi (of genera Aspergillus, Beaueria, Botryotrichum, Botrytis, Cladosporium, Epicoccum, Mortierella, Parengyodontium, Penicillium, and Trichoderma) in range of from 55.9% to 80.9% for certain tested isolates. The whole-genome sequencing and the analysis of genes included in the synthesis of bioactive secondary metabolites were also performed for these biocontrol strains as the necessary step in the development of efficient bioformulation (Dimkić et al., 2023).
In order to formulate and investigate the efficiency of the bioactive compounds on the development of biodeteriogenic fungi on cultural heritage, as well as eliminate potential negative effect compounds could have on structural and aesthetic integrity of treated works of art, next phase in research should always entail testing of bacterial metabolites on laboratory mock models in simulated conditions. However, data from this research phase are few and far between. In work by Silva et al. (2015) two months after treatment of Cladosporium sp.-infected mortar slabs with Bacillus sp. CCMI 1053 biocompound very low level of visual fungal proliferation was documented (via optical and scanning electron microscopy (SEM)) and no cracking or detachments were observed on the surface of the slabs. Energy dispersive X-ray analysis (EDX) revealed C, N and O elements confirming the presence of some organic material in the slab. Furthermore, five months after treatment with biocompounds and cell-free supernatant of Bacillus sp. CCMMI 1053 no pigment alteration, cracking or other structural impairments were documented on mural paintings fragments. In another work by Silva et al. (2018), marble slabs inoculated with Penicillium glandicola, a common fungi found in biodeteriorated artworks, were treated with Bacillus sp. CCLBH 1053 supernatant under in situ-controlled conditions and its efficiency evaluated via SEM-EDX and total fungal DNA reduction. Thirty days after biocompound application SEM-EDX analysis of treated slabs showed little evidence of P. glandicola growth and only presence of elements in marbles natural composition (Na,Cl, Ca), while fungal DNA amount was significantly lower compared to untreated slabs with mycelial growth inhibition percentage of almost 100%. In their latest research, one month after treatment of Ançã limestone slabs infested with fungi from Acremonium, Aspergillus and Penicillium genera, with lipopeptides BEVOTECH 11, 14 and 16, observations showed great capacity of these compounds to inhibit fungal proliferation (SEM) and decrease viable cells (MTT colorimetric assay). Used compounds were also completely safe, from heritage material standpoint, and did not induce any color, texture or structure alterations to limestone slabs (Rosado et al., 2019). Finally, really promissing results were obtained in simulated conditions with fungal infested laboratory models of mural painting treated with B. altitudinis 6-1 TSA and S. anulatus 1-3MM full cultures and supernatants (I. Dimkić, personal communication). Such promising results obtained with laboratory models indicate that highly effective Bacillus metabolites could in the future be applied in situ on various cultural heritage monuments made of stone, for example marble (Fig.3).
3. Antifungal metabolites of bacterial origin
Up to date literature overview has clearly pointed out Bacillus species as emerging promising alternative for cultural heritage treatment due to their ability to produce multitude of bioactive secondary metabolites, including ribosomally synthesized lanthipeptides (subtilin, entianin, ericins, etc.) with antibacterial activity and non-ribosomally synthesized peptides (bacilysin and rhizocticin) and lipopeptides (surfactin, fengycin, and iturin, to name a few) known for their antifungal properties (Caldeira et al., 2021). Many species of Bacillus genera, such as B. amyloliquefaciens, B. pumilus, and B. subtilis, are considered the main producers of antagonistic lipopeptides with some strains known to synthesize several lipopeptides. Bacillus-produced lipopeptides possess nontoxic mode of action, based on cell membrane breakdown of the target organism ensuing imbalanced movement of ions, damage and death of the cell, at the same time making it very difficult for target fungi to develop resistance which is a great advantage compared to commercial biocides (Silva et al., 2016a; Caldeira et al., 2021). These lipopeptide properties are highly compatible with their high biodegradability, non-harmful and environmentally friendly characteristics (Silva et al., 2019). Having in mind non-pathogenic nature of many Bacillus species and great physiological diversity, combined with easy cultivation, manipulation and high metabolite yield, makes production of their biocompounds in industrial bioreactors ideal solution for large scale in situ application on infested works of art (Caldeira et al., 2021).

Figure 3. Prototype of in situ biocontrol treatment of marble fungal infestation with Bacillus compounds.
4. Toxicological evaluations of bioactive compounds
To meet the public safety concern in regards to usage of bacteria as biocontrol agents, prior to any testing or application it is necessary to carry out screening and only use strains categorized as Generally Regarded as Safe (GRAS) by the U.S. Food and Drug Administration (USFDA). These are Biosafety Level 1 (BSL-1) low-risk microbes that are highly unlikely to cause disease in healthy adults and present minimal potential hazard to the environment. All biocontrol bacteria must be categorized as BSL-1 risk group. Bacillus subtilis and B. amyloliquefaciens are, for example, granted GRAS status by the USFDA which designates them as non-pathogenic and applicable as biopesticides (Usta et al., 2013).
Unidentified bioactive compounds produced by Bacillus species, proven in vitro to possess great potential to suppress fungal growth on historical artworks, were also shown to lack acute toxicity, motor, cognitive or sensorial alterations when applied to brine shrimp Artemia salina and Swiss mice in concentrations ten times higher compared to commercial biocide Preventol® (Silva et al., 2016a). Hence, good biocontrol properties against fungi of genera Alternaria, Fusarium and Penicillium, isolated from unnamed deteriorated mural paintings, combined with non-harmful and environmentally friendly characteristics, represent an excellent basis for utilization of these compounds for the development of bioformulation that are safe alternatives for treatment of fungal infested cultural heritage objects.
5. Future prospects
Based on the results from a limited number of per- formed preliminary studies beneficial bacteria, mostly of Bacillus genus, possess great potential to be utilized as a novel, low to no risk, selective and eco- friendly tool for sustainable and long-term suppres- sion of fungal infestation of works of art. Additional studies are necessary to evaluate potential risks and confirm the absence of any form of negative impact applied bacteria-based bioformulation might have on a structural or aesthetic integrity of treated artwork, determine the most adequate method and rhythm of application, evaluate the long-term effectiveness of applied treatment and possible tertiary colonisation by fungi, and determine the costs of its production compared to biocidal products and other treatments. If these studies yield positive results, novel eco-sus- tainable alternatives for mitigation of biodeteriora- tion problem could very well become groundbreak- ing new norm in conservation practice and replace commercial toxic compounds.
Funding: This research was supported by the Science Fund of the Republic of Serbia, PROMIS, [#GRANT No. 6066210, PROTECTA], and by the Ministry of Education, Science and Technological developments of the Republic of Serbia [[#GRANT No. 451-03- 47/2023-01/200178].
Informed Consent Statement: Not applicable. Data Availability Statement: Not applicable. Acknowledgments: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest.
References
- Bogaert, D., De Groot, R., & Hermans, P. W. (2004). Streptococcus pneumoniae colonisation: the key to pneumococcal disease. The Lancet. Infectious diseases, 4(3), 144–154.
- Allsopp, D. (2011). Worldwide wastage: the economics of biodeterioratio Microbiology Today, 38, 150-153.
- Caldeira, T. (2021). Green Mitigation Strategy for Cultural Heritage Using Bacterial Biocides. In E. Joseph (Ed.), Microorganisms in the Deterioration and Preservation of Cultural Heritage (pp. 137-154).
- Cappitelli, F., Cattò, , & Villa, F. (2020). The control of cultural heritage microbial deterioration. Microorganisms, 8(10), 1542.
- Caselli, , Pancaldi, S., Baldisserotto, C., Petrucci, F., Impallaria, A., Volpe, L., D’Accolti, M., Soffritti, I., Coccagna, M., Sassu, G., Bevilacqua, F., Volta, A., Bisi, M., Lanzoni, L., & Mazzacane, S. (2018). Characterization of biodegradation in a 17th century easel painting and potential for a biological approach. PLoS One, 13(12), e0207630.
- Dimkić, , Ćopić, M., Petrović, M., Stupar, M., Savković, Ž., Knežević, A., Subakov Simić, G., Ljaljević Grbić, M., & Unković, N. (2023). Bacteriobiota of the Cave Church of Sts. Peter and Paul in Serbia—Culturable and Non-Culturable Communities’Assessment in the Bioconservation Potential of a Peculiar Fresco Painting. International Journal of Molecular Sciences, 24, 1016.
- Fonseca, J., Pina, F., Macedo, M. F., Leal, N., Romanowska-Deskins, A., Laiz, L., Gómez Bolea, A., & Saiz-Jimenez, C. (2010). Anatase as an alternative application for preventing biodeterioration of mortars: Evaluation and comparison with other biocides. International Biodeterioration & Biodegradation, 64(5), 388-396.
- Fidanza, R., & Caneva, G. (2019). Natural biocides for the conservation of stone cultural heritage: A review. Journal of Cultural Heritage, 38, 271-286.
- Ljaljević Grbić, , Unković, N., Dimkić, I., Janaćković, P., Gavrilović, M., Stanojević, O., Stupar, M., Vujisić, Lj., Jelikić, A., Stanković, S., & Vukojević, J. (2018). Frankincense and myrrh essential oils and burn incense fume against micro-inhabitants of sacral ambients. Wisdom of the ancients?. Journal of Ethnopharmacology, 219, 1-14.
- Martin-Sanchez, P. , Nováková, A., Bastian, F., Alabouvette, C., & Saiz-Jimenez, C. (2012). Use of biocides for the control of fungal outbreaks in subterranean environments: The case of the Lascaux Cave in France. Environmental Science & Technology, 46(7), 3762-3770.
- Nugari, P., & Salvadori, O. (2003). Biodeterioration control of cultural heritage: Methods and Products. In C. Saiz-Jimenez (Ed.), Molecular Biology and Cultural Heritage (pp. 233–242).
- Caldeira, T. (2021). Green Mitigation Strategy for Cultural Heritage Using Bacterial Biocides. In E. Joseph (Ed.), Microorganisms in the Deterioration and Preservation of Cultural Heritage (pp. 137-154).
- Kakakhel, A., Wu, F., Gu, J. D., Feng, H., Shah, K., & Wang, W. (2019). Controlling biodeterioration of cultural heritage objects with biocides: A review. International Biodeterioration & Biodegradation, 143, 104721.
- Palla, F., Bruno, , Mercurio, F., Tantillo, A., & Rotolo, V. (2020). Essential oils as natural biocides in conservation of cultural heritage. Molecules, 25(3), 730.
- Rosado, T., Silva, M., Dias, L., Candeias, A., Gil, M., Mirão, J., Pestana, J., & Caldeira, A. T. (2017). Microorganisms and the integrated conservation-intervention process of the renaissance mural paintings from Casas Pintadas in Évora–Know to act, act to preserve. Journal of King Saud University-Science, 29(4), 478-486.
- Rosado, T., Santos, R., Silva, , Galväo, A., Miräo, J., Candeias, A., & Caldeira, A. T. (2019). Mitigation approach to avoid fungal colonisation of porous limestone. International Journal of Conservation Science, 10(1), 3-14.
- Salvador, , Silva, M., Rosado, T., Vaz Freire, R., Bordalo, R., Candeias, A., & Caldeira, A. T. (2016). Biodeterioration of easel paintings: development of new mitigation strategies. Conservar Património, 23, 119-124.
- Sasso, , Scrano, L., Ventrella, E., Bonomo, M. G., Crescenzi, A., Salzano, G., & Bufo, S. A. (2013). Natural biocides to prevent the microbial growth on cultural heritage. Built Heritage, 1, 1035-1042.
- Scheerer, , Ortega‐Morales, O., & Gaylarde, C. (2009). Microbial deterioration of stone monuments—an updated overview. Advances in Applied Microbiology, 66, 97-139.
- Silva, , Rosado, T., Teixeira, D., Candeias, A., & Caldeira, A. T. (2015). Production of green biocides for cultural heritage. Novel biotechnological solutions. International Journal of Conservation Science, 6, 519- 530.
- Silva, , Salvador, C., Candeias, M. F., Teixeira, D., Candeias, A., & Caldeira, A. T. (2016a). Toxicological assessment of novel green biocides for cultural heritage. International Journal of Conservation Science, 7(1), 265-272.
- Silva, , Pereira, A., Teixeira, D., Candeias, A., & Caldeira, A. T. (2016b). Combined use of NMR, LC- ESI-MS and antifungal tests for rapid detection of bioactive lipopeptides produced by Bacillus. Advances in Microbiology, 6(10), 788-796.
- Silva, , Rosado, T., Teixeira, D., Candeias, A., & Caldeira, A. T. (2017). Green mitigation strategy for cultural heritage: bacterial potential for biocide production. Environmental Science and Pollution Research, 24(5), 4871-4881.
- Silva, , Rosado, T., Gonzalez-Pérez, M., Gobbo, D., Teixeira, D., Candeias, A., & Caldeira, A. T. (2018). Production of antagonistic compounds by Bacillus sp. with antifungal activity against heritage contaminating fungi. Coatings, 8(4), 123.
- Silva, , Rosado, T., da Silva, Z. L., Nóbrega, Y., Silveira, D., Candeias, A., & Caldeira, A. T. (2019). Green bioactive compounds: mitigation strategies for cultural heritage. Conservation Science in Cultural Heritage, 19, 133-142.
- Sterflinger, (2010). Fungi: their role in deterioration of cultural heritage. Fungal Biology Reviews, 24(1-2), 47-55.
- Sterflinger, , & Piñar, G. (2013). Microbial deterioration of cultural heritage and works of art—tilting at windmills?. Applied Microbiology and Biotechnology, 97(22), 9637-9646.
- Tayel, A., Ebeid, M. M., El Sawy, E., & Khalifa, S. A. (2016). Fungicidal effects of plant smoldering fumes on archival paper-based documents. Restaurator, 37(1), 15-28.
- Usta, (2013). Microorganisms in biological pest control—a review (Bacterial toxin application and effect of environmental factors). In M. Silva-Opps (Ed.), Current Progress in Biological Research (pp. 287- 317).
- Varnai, V. , Macan, J., Ljubičić Ćalušić, A., Prester, L., & Kanceljak Macan, B. (2011). Upper respiratory impairment in restorers of cultural heritage. Occupational Medicine, 61(1), 45-52.
