1. Introduction
Urinary tract infections (UTIs) are among the most frequent infectious diseases encountered in primary care and represent a major reason for antibiotic prescriptions, second only to respiratory tract infections (Sanchez et al. 2023). In recent years, the approach to diagnosing and managing UTIs has undergone substantial advancement, largely due to the growing threat of antimicrobial resistance (AMR) and the need for tailored, evidence-based treatment strategies. Within this evolving landscape, microbiological diagnostics have become pivotal, not only for confirming infection but also for directing appropriate therapy, detecting resistance mechanisms, and reinforcing antimicrobial stewardship efforts.
The urine culture is considered the gold standard for diagnosing UTIs, allowing for rational and focused therapy. However, the reliability of this diagnostic tool relies heavily on proper sampling, swift processing, and accurate laboratory techniques.
In this review, we aimed to explore the multifaceted role of the microbiology laboratory, from the pre-analytical to the result interpretation phase.
2. Sampling, transport, and laboratory processing
Accurate microbiological diagnosis of urinary tract infections (UTIs) depends on strict adherence to protocols during urine collection, transport, and laboratory handling. Errors in any of these steps can lead to contamination, false-positive results, or missed infections, ultimately compromising patient care.
2.1 Urine sampling and transport
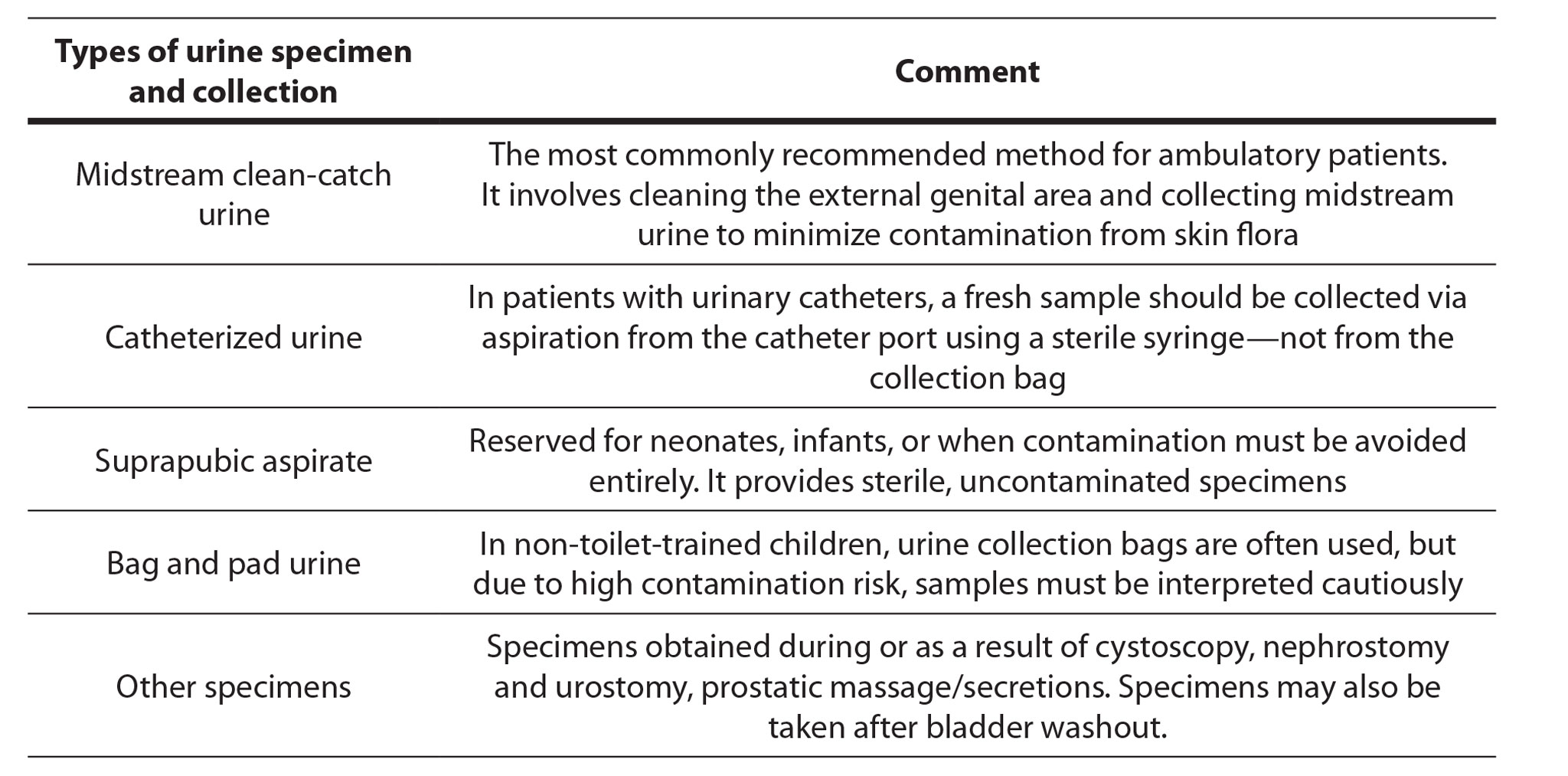
Accurate UTIs diagnosis begins with appropriate urine collection in a sterile container. Midstream urine (MSU) and clean-catch urine are the most commonly collected specimens and are recommended for routine use. The types of urine specimen and collection are presented In Table 1 (Public Health England, 2019).
Urine should ideally be transported to the laboratory within 2 hours of collection. Delays can lead to overgrowth of contaminants and inaccurate colony counts. If immediate transport is not possible, urine should be refrigerated at 4°C for up to 24 hours to preserve sample integrity. Commercial urine transport systems containing boric acid or similar preservatives are useful for maintaining sample quality during delayed transport.
2.2 Laboratory processing and report interpretation
Laboratory investigation of UTIs normally involves microscopy (or an alternative method of measuring cellular components) and quantitative culture (or an alternative non-culture method such as a semi-automated urine analyser). Culture is typically performed using a calibrated loop (commonly 1 µL or 10 µL) to inoculate culture media, most often chromogenic agar, cystine–lactose–electrolyte-deficient (CLED) agar, or blood agar. Plates are incubated aerobically at 35–37°C for 16–24 hours. In specific clinical contexts, extended incubation or anaerobic conditions may be applied.
Interpretation of bacterial growth is based on colony count thresholds, clinical presentation, and collection method. Some protocols adopt sex-specific thresholds of ≥10⁵ CFU/mL for women and ≥10³ CFU/mL for men (Gupta, et al. 2011; Hryniewicz, et al. 2013). In specific patient groups, counts between 105 cfu/mL and 102 cfu/mL may be significant (National Institute for Health and Care Excellence, 2022). Mixed growth (≥2 species) suggests sample contamination, especially when commensal microbiota is present ( Public Health England, 2019)
Identification is typically achieved using conventional biochemistry or Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry, while antimicrobial susceptibility testing is performed and interpreted according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) or Clinical and Laboratory Standards Institute (CLSI) guidelines (European Committee on Antimicrobial Susceptibility Testing, 2025; Clinical and Laboratory Standards Institute, 2025). New diagnostic technologies, including PCR panels and automated urine analyzers, enable quicker result turnaround and have the potential to shorten the time to targeted treatment. Although urine culture is still necessary for determining antimicrobial susceptibility, these rapid tools help close the gap between the onset of clinical symptoms and the start of appropriate therapy.
Table 1. Types of urine specimens and their relevance in diagnostic testing
The most commonly isolated uropathogens include Escherichia coli (70–80%), Klebsiella spp., and Proteus mirabilis (Gupta, et al. 2011; Hryniewicz, et al. 2013). Accurate susceptibility profiling is crucial to guide therapy and minimize the spread of resistant strains. Resistance mechanisms such as extended-spectrum β-lactamase (ESBL) production or fluoroquinolone resistance must be reported promptly to inform clinical decisions.
Prompt and well-interpreted laboratory reports, aligned with the patient’s clinical condition, are crucial to prevent failures of empirical therapy (Hryniewicz, et al. 2013).
- Antimicrobial resistance of major UTIs pathogens
- coli remains the leading cause of UTIs but shows high resistance to commonly used antibiotics such as ampicillin, ceftriaxone, and fluoroquinolones, while carbapenem resistance remains low (Jalil and Atbee, 2022).
- pneumoniae is frequently multidrug-resistant, with high rates of resistance to β-lactams and fluoroquinolones, largely due to ESBL production (Silva et al. 2022).
Enterococcus spp. are increasingly detected in complicated UTIs and often show resistance to penicillin and fluoroquinolones, though linezolid and tigecycline remain effective (Mareș et al. 2024).
Rising antimicrobial resistance among these pathogens poses a major challenge for empirical UTI treatment, highlighting the need for local surveillance and antibiotic stewardship.
4. Therapeutic guidelines – national and international
Serbian national guidelines recommend the following: Rational Use of Antibiotics recommends fluoroquinolones, fosfomycin, and cephalosporins as first-line treatments for acute cystitis, with second-line options including amoxicillin/clavulanic acid, cephalosporins, and trimethoprim sulphametoxasole (advised if E. coli resistance is below 20%) (Ministry of Health, Republic of Serbia, 2018). The other guideline, Management of uncomplicated UTIs suggests fosfomycin trometamol, nitrofurantoin, and pivmecillinam (although not licensed in Serbia) as first-line treatments, with fluoroquinolones and trimethoprim sulphametoxasole (if resistance is low) as alternatives (Ministry of Health, Republic of Serbia, 2022). For prostatitis, suggesting broad-spectrum penicillins, third-generation cephalosporins, and a combination of fluoroquinolones and aminoglycosides (Ministry of Health, Republic of Serbia, 2022). For acute pyelonephritis, both Serbian guidelines recommend cephalosporins, fluoroquinolones, and aminoglycosides.
European Association of Urology (EAU) and American Infectious Diseases Society of America (IDSA) guidelines promote narrow-spectrum agents, emphasizing the avoidance of fluoroquinolones due to safety concerns and resistance patterns (Bachmann, 2022; U.S. and global perspectives, Bonkat, 2023). Across all systems, individualized therapy remains a central theme (Gupta, 2011).
5. Local resistance and the value of the ex juvantibus approach
Effective management of UTIs increasingly depends on up‑to‑date, locale‑specific AMR surveillance. The ex juvantibus approach, meaning empirical treatment based on prior local antibiogram data, clinician experience and patient’s previous therapeutic responses, can significantly enhance therapeutic success, especially in cases of recurrent infection, atypical pathogens or in geographic areas with high AMR prevalence. Microbiological laboratories play a pivotal role in this process by generating accurate, context‑specific resistance profiles (rather than relying solely on national or broad‑scale trends), thereby enabling individualized therapy tailored to the local microbial ecology.
Recent studies support this shift in paradigm: In Hungary, a long‑term study of UTI bacterial spectra and resistance between 2012‑2023 demonstrated meaningful shifts in the prevalence of key pathogens and their resistance profiles, further emphasising the necessity of regionally calibrated antibiograms (Fehér et al. 2025). Moreover, recent narrative review evidence highlights that for urological infections the choice of antibiotic “should be based on local level of resistance of uropathogens (Kulchavenya, 2020).
Accordingly, the ex juvantibus approach becomes a bridge between empirical prescribing and culture‑guided therapy: while awaiting or in absence of definitive microbiology results, the clinician uses high‑quality local data + prior patient history (e.g., prior UTI with known resistant organism) to guide initial antibiotic choice. This strategy helps to maximize the likelihood of early effective therapy, while stewardship programmes and rapid diagnostics aim to refine or switch therapy as more precise data become available.
In practice, this means:
- Local antibiograms must be updated regularly (preferably annually or semi‑annually) and stratified by setting (community vs hospital, long‑term care, outpatient vs catheterised vs non‑catheterised).
- Laboratories should report not only the “resistant/susceptible” status, but also prevalence of particular mechanisms (e.g., ESBL producers, fluoroquinolone resistance) and note significant shifts over time.
- Clinicians should document prior infectious episodes, prior antibiotic exposures and known colonisation or infection with resistant organisms, and use that as part of initial empiric therapy decision‑making.
- The empiric choice should then be refined as culture & susceptibility results return, promoting targeted therapy, shorter duration, minimized broad-spectrum use and thereby reducing onward selection of resistant organisms.
By embedding this collaborative, data‑driven approach into everyday practice, we move from “blind” empiricism toward a model of informed empiricism, which ultimately supports both individual patient outcomes and collective antimicrobial stewardship.
6. Interdisciplinary collaboration
The clinical impact of laboratory reports is significantly amplified when microbiologists and clinicians engage in active, interdisciplinary collaboration. Rather than simply generating test results, microbiologists contribute expert interpretation, placing microbiological data within the broader context of emerging resistance patterns, local epidemiology, and patient-specific clinical factors.
This bidirectional communication becomes especially critical in complex cases, such as recurrent urinary tract infections, atypical presentations, or infections unresponsive to standard therapy. In such scenarios, laboratory data alone may be insufficient; nuanced interpretation and discussion between disciplines can uncover alternative diagnoses, adjust therapeutic strategies, or prompt additional testing.
Integrating microbiologists into clinical decision-making processes ensures that diagnostic insights are fully leveraged to inform timely, precise, and personalized treatment. This collaborative approach transforms the laboratory from a passive service provider into an active clinical partner, ultimately improving patient outcomes and promoting antimicrobial stewardship.
7. Conclusion
Effective treatment of UTIs requires more than empirical prescribing; it demands coordinated, evidence-based decision-making anchored in high-quality microbiological diagnostics. Accurate specimen collection, precise identification of causative pathogens, and reliable antimicrobial susceptibility testing are fundamental to for guiding appropriate therapy. These diagnostic pillars not only inform targeted treatment but also help mitigate the risk of AMR. Crucially, close collaboration between clinical microbiologists and healthcare providers ensures that laboratory data are not just reported, but meaningfully interpreted and applied in the clinical context. This interdisciplinary partnership bridges the gap between bench and bedside, facilitating timely, patient-specific therapeutic decisions. Within this integrated model, microbiological reporting evolves from a passive confirmation tool into a dynamic instrument for clinical guidance, actively shaping the course of treatment.
Conflict of interest: The authors declare no conflicts of interest.
References
Bachmann, L. H., et al. (2022). Management of urinary tract infections: U.S. and global perspectives. Clinical Infectious Diseases, 75(Suppl_1), S36–S42.
Bonkat, G., et al. (2023). EAU Guidelines on Urological Infections. European Association of Urology. Retrieved from https://uroweb.org/guidelines/urological-infections
Clinical and Laboratory Standards Institute. (2025). Performance standards for antimicrobial susceptibility testing (35th ed., CLSI supplement M100). Clinical and Laboratory Standards Institute.
European Committee on Antimicrobial Susceptibility Testing. (2025). Breakpoint tables for interpretation of MICs and zone diameters (Version 15.0). https://www.eucast.org
Fehér, Á. M., Safikhani, M., Bajory, Z., Lázár, A., Burián, K., Rárosi, F., & Köves, B. (2025). Urinary bacterial spectrum and antibiotic resistance trends at a Urology Clinic in Hungary between 2012 and 2023. International urology and nephrology, 10.1007/s11255-025-04683-z. Advance online publication. https://doi.org/10.1007/s11255-025-04683-z
Gupta, K., et al. (2011). International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women. Clinical Infectious Diseases, 52(5), e103–e120.
Hryniewicz, W., et al. (2013). Urinary tract infections: Epidemiology, diagnostics, treatment, and prophylaxis. Postępy Nauk Medycznych, 26(9), 749–753.
Jalil, M. B., & Al Atbee, M. Y. N. (2022). The prevalence of multiple drug resistance Escherichia coli and Klebsiella pneumoniae isolated from patients with urinary tract infections. Journal of clinical laboratory analysis, 36(9), e24619.
Kulchavenya E. (2020). The best rules for antimicrobial stewardship in urogenital tract infections. Current opinion in urology, 30(6), 838–844. https://doi.org/10.1097/MOU.0000000000000817
Mareș, C., Petca, R. C., Popescu, R. I., Petca, A., Mulțescu, R., Bulai, C. A., Ene, C. V., Geavlete, P. A., Geavlete, B. F., & Jinga, V. (2024). Update on Urinary Tract Infection Antibiotic Resistance-A Retrospective Study in Females in Conjunction with Clinical Data. Life (Basel, Switzerland), 14(1), 106. https://doi.org/10.3390/life14010106
Ministry of Health, Republic of Serbia. The National Guideline of Good Clinical Practice: The rational Use of Antibiotics. 2018. Available: https://www.zdravlje.gov.rs/view_file.php?file_id=527&cache=sr
Ministry of Health, Republic of Serbia. The National Guideline of Good Clinical Practice: Management of Uncomplicated UTIs. 2022. Available: https://www.zdravlje.gov.rs/view_file.php?file_id=2349&cache=sr)
National Institute for Health and Care Excellence. (2022). Urinary tract infection in under 16s: Diagnosis and management (NICE Guideline NG224). https://www.nice.org.uk/guidance/ng224
Public Health England. (2019). Bacteriology B 41: Investigation of urine (Issue no. 8.7, p. 16 of 51). UK Standards for Microbiology Investigations. https://www.gov.uk/uk-standards-for-microbiology-investigations
Sánchez, X., Latacunga, A., Cárdenas, I., Jimbo-Sotomayor, R., & Escalante, S. (2023). Antibiotic prescription patterns in patients with suspected urinary tract infections in Ecuador. PloS one, 18(11), e0295247. https://doi.org/10.1371/journal.pone.0295247
Silva, A., Costa, E., Freitas, A., & Almeida, A. (2022). Revisiting the Frequency and Antimicrobial Resistance Patterns of Bacteria Implicated in Community Urinary Tract Infections. Antibiotics (Basel, Switzerland), 11(6), 768.
Tchesnokova, V., et al. (2019). Predicting antibiotic resistance in uropathogens using local and global epidemiology. Journal of Antimicrobial Chemotherapy, 74(7), 1894–1901.
Zdziarski, J., et al. (2021). Ex juvantibus approach in recurrent urinary tract infections. Antibiotics, 10(8), 945.